
GAMMAGARD S/D 10 G, POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION

Cómo usar GAMMAGARD S/D 10 G, POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es GAMMAGARD S/D y para qué se utiliza
- Qué necesita saber antes de empezar a usar GAMMARD S/D
- Si esto ocurriera, espere hasta que las reacciones hayan desaparecido.
- Cómo usar GAMMAGARD S/D
- Posibles efectos adversos
- Conservación de GAMMAGARD S/D
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
GAMMAGARD S/D 10 g, polvo y disolvente para solución para perfusión
Inmunoglobulina humana normal
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es GAMMAGARD S/D y para qué se utiliza
- Qué necesita saber antes de empezar a usar GAMMAGARD S/D
- Cómo usar GAMMAGARD S/D
- Posibles efectos adversos
- Conservación de GAMMAGARD S/D
- Contenido del envase e información adicional
1. Qué es GAMMAGARD S/D y para qué se utiliza
GAMMAGARD S/D pertenece a una clase de medicamentos llamados inmunoglobulinas. Estos medicamentos contienen anticuerpos humanos, presentes también en la sangre. Los anticuerpos ayudan a combatir las infecciones. Los medicamentos como GAMMAGARD S/D se utilizan cuando no tienen anticuerpos suficientes en la sangre. Estos pacientes suelen padecer infecciones con frecuencia. GAMMAGARD S/D también se puede utilizar cuando se necesiten anticuerpos adicionales para la cura de determinados trastornos inflamatorios (enfermedades autoinmunes).
GAMMAGARD S/D10 g se utiliza para
Tratamiento de pacientes que no tienen anticuerpos suficientes (tratamiento de reposición). Hay cinco grupos:
- Pacientes con una falta congénita de producción de anticuerpos (síndromes de inmunodeficiencia primaria (IDP)) como:
- agammaglobulinemia o hipogammaglobulinemia congénitas,
- inmunodeficiencia común variable,
- inmunodeficiencias combinadas graves,
- Síndrome de Wiskott-Aldrich
- Pacientes con un cáncer de la sangre (leucemia linfocítica crónica) que provoque una falta de producción de anticuerpos e infecciones recurrentes cuando el tratamiento preventivo con antibióticos haya fallado.
3 Pacientes con un cáncer de médula ósea (mieloma múltiple) y falta de producción de anticuerpos con infecciones recurrentes en los que ha fallado la repuesta a la vacuna frente a determinadas bacterias (neumococos).
4 Niños y adolescentes (0 a 18 años) con SIDA de nacimiento e infecciones frecuentes.
5 Pacientes con una baja producción de anticuerpos después de un trasplante de células de médula ósea de otra persona.
Tratamiento de pacientes con determinados trastornos inflamatorios (efecto inmunomodulador). Hay tres grupos:
- Pacientes que no tienen suficientes plaquetas en la sangre (púrpura trombocitopénica idiopática/primaria, PTI) y con un alto riesgo de hemorragia o que vayan a ser sometidos a una intervención quirúrgica próximamente.
- Pacientes con una enfermedad que provoca la inflamación múltiple de diversos órganos del cuerpo (Enfermedad de Kawasaki).
- Pacientes con una enfermedad que se caracteriza por la inflamación múltiple de los nervios de todo el cuerpo (Síndrome de Guillain Barré).
2. Qué necesita saber antes de empezar a usar GAMMARD S/D
No useGAMMAGARDS/D
- Si es alérgico (hipersensible) a las inmunoglobulinas o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si tiene una deficiencia de inmunoglobulina A. Puede tener anticuerpos anti-inmunoglobulina A en la sangre. GAMMAGARD S/D contiene cantidades muy pequeñas de inmunoglobulina A y usted puede desarrollar una reacción alérgica.
Advertencias y precauciones
Período de control requerido durante la perfusión
- Usted será controlado atentamente durante el periodo de perfusión de GAMMAGARD S/D para evitar que sufra una reacción alérgica. Su médico se asegurará de que la velocidad de perfusión de GAMMAGARD S/D es la adecuada en su caso.
Puede existir un riesgo mayor de efectos adversos:
- si GAMMAGARD S/D se administra a una velocidad alta,
- si padece un trastorno que se caracteriza por un nivel bajo de anticuerpos en la sangre (hipo- o agammaglobulinemia),
- si no ha recibido este medicamento antes o
- si ha transcurrido un largo periodo (por ejemplo, varias semanas) desde la última vez que le fue administrado.
En estos casos, usted será controlado estrechamente durante la perfusión y durante una hora después de que la perfusión haya terminado ya que puede existir mayor riesgo de efectos adversos.
Si ya ha recibido GAMMAGARD S/D recientemente, sólo será observado durante la perfusión y durante, al menos, 20 minutos después de la perfusión.
Cuándo es necesario detener o disminuir la velocidad de la perfusión
En casos raros, su organismo puede estar sensibilizado a medicamentos que contienen anticuerpos. Esto puede ocurrir sobre todo si presenta una deficiencia de inmunoglobulina A. En estos casos raros, pueden padecer reacciones alérgicas como un descenso brusco de la tensión arterial o un shock, incluso si ha recibido anteriormente un tratamiento con medicamentos que contienen anticuerpos.
- Si usted nota alguno de los síntomas siguientes, informe inmediatamente a su médico o enfermera:
- Sibilancias repentinas, dificultad al respirar u opresión en el pecho
- Dolor de cabeza
- Fiebre
- Hinchazón de los párpados, cara, labios o vasos sanguíneos
- Bultos o manchas rojas con picor en la piel
- Picor por todo el cuerpo
Dependiendo de la decisión de su médico se puede disminuir la velocidad o parar la perfusión.
Grupos de pacientes especiales
Su médico debe extremar las precauciones si usted padece sobrepeso, es de edad avanzada, es diabético, se encuentra inmovilizado, si utiliza estrógenos, tiene una sonda vascular permanente o tiene tendencia a padecer trombosis.
Su médico le observará cuidadosamente si usted tiene:
- tensión arterial alta
- el volumen de sangre bajo (hipovolemia)
- aumento de la viscosidad de la sangre o problemas en los vasos sanguíneos (enfermedades vasculares incluyendo gasto cardíaco o episodios trombóticos)
- coagulación en exceso o trastornos de coagulación.
En estos casos, las inmunoglobulinas pueden aumentar el riesgo de infarto de miocardio, accidente cerebrovascular, embolia pulmonar o trombosis venosa profunda, aunque en muy raros casos.
Informe a su médico si es diabético.
Este medicamento contiene glucosa. GAMMAGARD S/D no contiene sacarosa ni maltosa.
Los pacientes con diabetes mellitus deben tener en cuenta que una solución al 5% (50 mg/ml) de GAMMAGARD S/D contiene 400 mg de glucosa por gramo de IgG. Un paciente de 70 kg que recibe una dosis de 1 g/kg de IgG recibiría 28 gramos de glucosa o 112 calorías. Esto puede afectar a su nivel de azúcar en sangre.
Su médico tendrá también un cuidado especial
- si tiene o ha tenido problemas con sus riñones
- si recibe medicamentos que puedan dañar sus riñones (medicamentos nefrotóxicos), ya que existe una probabilidad muy rara de fallo renal agudo. Informe a su médico si tiene o ha tenido problemas renales.
El contenido de proteínas puede aumentar provocando una mayor viscosidad de la sangre
Información sobre el material original deGAMMAGARDS/D
GAMMAGARD S/D se fabrica a partir de plasma humano (la parte líquida de la sangre).Cuando los medicamentos se elaboran a partir de sangre o plasma humanos, se deben adoptar un número de medidas para prevenir una posible transmisión de infecciones a los pacientes. Estas medidas incluyen una selección cuidadosa de los donantes de sangre y plasma para garantizar la exclusión de donantes con riesgo de padecer infecciones y el análisis de cada donación y mezcla de plasmas para detectar posibles virus o infecciones. Los fabricantes de estos productos incluyen además una serie de etapas en el procesamiento de la sangre o el plasma que pueden inactivar o eliminar los virus. A pesar de estas medidas, cuando se administran medicamentos preparados a partir de sangre o plasma humanos, no se puede excluir totalmente la posibilidad de transmisión de infecciones. Esto es aplicable también a los virus desconocidos o emergentes y a otros tipos de infecciones.
Las medidas adoptadas se consideran eficaces para los virus encapsulados como el virus de la inmunodeficiencia humana (VIH), el virus de la hepatitis B (VHB) y el virus de la hepatitis C (VHC), y para el virus no encapsulado de la hepatitis A (VHA). Las medidas adoptadas pueden tener un valor limitado para virus no encapsulados tales como el parvovirus B19.
Las inmunoglobulinas no se han asociado con infecciones por hepatitis A o parvovirus B19 posiblemente porque los anticuerpos frente a estas infecciones, que están presentes en el producto, son protectores.
Se recomienda que cada vez que se le administre GAMMAGARD S/D, se deje constancia del nombre del medicamento y número de lote administrado para mantener un registro de los lotes utilizados.
Uso de GAMMAGARDS/Dcon otros medicamentos
Comunique a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento o si ha sido vacunado en las últimas seis semanas.
La perfusión de inmunoglobulinas como GAMMAGARD S/D puede alterar la eficacia de algunas vacunas de virus vivos como la del sarampión, rubéola, paperas y varicela. Por tanto, tras la administración de estos medicamentos, puede que tenga que esperar hasta 3 meses antes de recibir una vacuna de virus vivos atenuada. Puede que tenga que esperar hasta 1 año tras recibir inmunoglobulinas antes de la administración de la vacuna contra el sarampión.
Efectos sobre los análisis de sangre
GAMMAGARD S/D contiene una amplia variedad de anticuerpos diferentes, algunos de los cuales pueden interferir con los análisis de sangre. Si se le realiza un análisis de sangre, por favor informe al analista o a su médico de que se ha administrado GAMMAGARD S/D.
La administración de Gammagard S/D puede dar lugar a lecturas de falsos positivos en las pruebas que dependen de la detección de beta-D-glucanos para el diagnóstico de infecciones fúngicas; esto puede perdurar durante las semanas siguientes a las perfusión del producto.
Embarazo,lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
- No se han realizado ensayos clínicos con GAMMAGARD S/D en mujeres embarazadas o en periodo de lactancia. Los años de experiencia clínica con medicamentos que contienen anticuerpos han demostrado que no deben esperarse efectos perjudiciales durante el embarazo ni para el niño.
- Si usted está en periodo de lactancia, los anticuerpos de GAMMAGARD S/D pueden encontrarse en la lecha materna. Por tanto, su bebé puede estar protegido frente a ciertas infecciones.
- Los efectos de GAMMAGARD S/D sobre la fertilidad no se han establecido.
Conducción y uso de máquinas
Los pacientes pueden experimentar reacciones (por ejemplo, mareo o náuseas) durante el tratamiento con GAMMAGARD S/D que podrían afectar a la capacidad de conducir y utilizar máquinas.
Si esto ocurriera, espere hasta que las reacciones hayan desaparecido.
Gammagard S/D 10 gcontienesodio y glucosa
Este medicamento contiene 668 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada vial. Esto equivale al 34 % de la ingesta diaria máxima de sodio recomendada para un adulto.
Este medicamento contiene glucosa. Los pacientes con diabetes mellitus deben tener en cuenta que una solución al 5% (50 mg/ml) de GAMMAGARD S/D contiene 400 mg de glucosa por gramo de IgG. Un paciente de 70 kg que recibe una dosis de 1 g/kg de IgG recibiría 28 gramos de glucosa lo que puede afectar a su nivel de azúcar en sangre.
3. Cómo usar GAMMAGARD S/D
GAMMAGARD S/D es para administración intravenosa (inyección en vena). Le será administrado por su médico o enfermera. La dosis y la frecuencia de la perfusión pueden variar dependiendo de su situación y peso corporal.
Al comienzo de la perfusión usted recibirá GAMMAGARD S/D a una velocidad baja. Su médico puede aumentar gradualmente la velocidad de perfusión dependiendo de si usted lo tolera bien.
Uso en niños
En niños (0 a 18 años) se utilizan las mismas indicaciones, dosis y frecuencia de perfusión que en los adultos.
Si usa másGAMMAGARDS/Ddel que debe
Si recibe más GAMMAGARD S/D del que debiera, la sangre se puede espesar (hiperviscosidad). Cuanto más se espese la sangre, más difícil es su transporte a través de los vasos de su organismo, por tanto, se conducirá menos cantidad de oxígeno a los órganos vitales, como el cerebro, pulmones, etc. Esto puede ocurrir sobre todo si es un paciente de riesgo (por ejemplo, un paciente mayor o un paciente con problemas renales o cardíacos). Asegúrese de tomar los líquidos adecuados para no deshidratarse e informe a su médico si tiene problemas de salud.
En caso de sobredosis o administración accidental, consultar al Servicio de Información Toxicológica. Teléfono 915 620 420.
4. Posibles efectos adversos
Al igual que todos los medicamentos, GAMMAGARD S/D puede producir efectos adversos, aunque no todas las personas los sufran. No obstante, los posibles efectos adversos se pueden reducir al disminuir la velocidad de perfusión.
Los siguientes efectos adversos pueden ocurrir generalmente después del tratamiento con inmunoglobulinas (medicamentos como GAMMAGARD S/D):
- Frecuentes (pueden afectar hasta 1 de cada 10 personas): escalofríos, dolor de cabeza, fiebre, vómitos, náuseas.
- Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): leve dolor de la parte inferior de la espalda.
- Raros (pueden afectar hasta 1 de cada 1000 personas): casos de descenso brusco de la tensión arterial, síntomas parecidos al eccema (reacciones cutáneas transitorias).
- Frecuencia no conocida (no puede estimarse a partir de los datos disponibles): reacciones alérgicas, incluso en pacientes que no han presentado reacciones a las perfusiones anteriores; inflamación temporal de las membranas del cerebro (meningitis aséptica reversible); reducción temporal del número de glóbulos rojos en sangre; aumentos transitorios de los valores de la función hepática (transaminasas) y aumento del contenido de creatinina en sangre y fallo renal; formación de coágulos sanguíneos en las venas que han tenido como resultado un ataque al corazón, accidente cerebrovascular, daño en los pulmones y trombosis venosa profunda; dolor en las articulaciones; tensión arterial baja.
A continuación se describen los efectos adversos que algunos pacientes han notificado con GAMMAGARD S/D en los ensayos clínicos y los efectos adversos notificados durante la experiencia postcomercialización:
- Muy frecuentes (pueden afectar a más de 1 de cada 10 personas): ninguno.
- Frecuentes (pueden afectar hasta 1 de cada 10 personas): dolor de cabeza, enrojecimiento, náuseas, vómitos, fatiga, escalofríos, fiebre.
- Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): gripe, ansiedad, agitación, somnolencia anormal, vista borrosa, sentir los latidos del corazón, dificultad al respirar, sangrar por la nariz, diarrea, dolor en la parte superior de la tripa, malestar de estómago, inflamación de la boca, picor, ronchas en la piel, sudor frío, exceso de sudoración, dolor de espalda, calambres musculares, dolor de los brazos y piernas, dolor en el pecho, malestar en el pecho, sensación anormal, sensación de frío, sensación de calor, síntomas parecidos a los de la gripe, enrojecimiento en el lugar de la inyección, salida del medicamento de la vía en el lugar de la inyección, dolor en el lugar de la inyección, sensación de náuseas/vómitos, dolor, tensión arterial alta, alteraciones de la tensión arterial, pérdida de apetito.
- Frecuencia no conocida (no puede estimarse a partir de los datos disponibles): inflamación de las membranas del cerebro no causada por una infección bacteriana, destrucción de glóbulos rojos, disminución de la cantidad de glóbulos rojos, disminución de la cantidad de plaquetas, inflamación de los nódulos linfáticos, reacciones alérgicas de todo tipo de gravedad como shock alérgico, nerviosismo, mareo, sensación anormal en la piel, temblor involuntario, ataques, hemorragia cerebral, accidente cerebrovascular (transitorio), migraña, pérdida del conocimiento, intolerancia a la luz, alteración visual, dolor en el ojo, oclusión del vaso sanguíneo central del ojo, ataque cardíaco, coloración azulada de la piel, aumento del ritmo cardíaco, disminución del ritmo cardíaco, tensión arterial alta, palidez, tensión arterial baja, inflamación de las venas, oclusión de los vasos sanguíneos, tos, opresión de garganta, disminución del nivel de oxígeno de la sangre, hiperventilación, pitidos en el pecho, espasmos en las vías respiratorias, oclusión de los vasos sanguíneos del pulmón, líquido en el pulmón, alteración de la digestión, dolor abdominal, inflamación del hígado (no transmisible), enrojecimiento de la piel, erupciones cutáneas, inflamación de la piel, inflamación alérgica de las capas profundas de la piel, dolor muscular y de las articulaciones, fallo renal, debilidad generalizada, hinchazón de los tejidos del cuerpo, reacciones en el lugar de la inyección y perfusión, resultado positivo del test de Coombs.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de GAMMAGARD S/D
- Mantener fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día de ese mes.
- No usar si se observan partículas o decoloración.
- No conservar a temperatura superior a 25ºC.
- No congelar.
Mantener el envase en el embalaje exterior para protegerlo de la luz.
6. Contenido del envase e información adicional
Composición deGAMMAGARD S/D
El principio activo de GAMMAGARD S/D es inmunoglobulina humana normal.
GAMMAGARD S/D se puede reconstituir con agua esterilizada para preparaciones inyectables como una solución de proteína al 5 % (50 mg/ml) o al 10 % (100 mg/ml). Al menos, el 90% es inmunoglobulina G (IgG).
Los demás componentes son albúmina humana, glicina, cloruro de sodio y glucosa monohidrato.
Aspecto del producto y contenido del envase
GAMMAGARD S/D es un polvo liofilizado de color blanco o débilmente amarillo, sustancialmente libre de partículas extrañas visibles. GAMMAGARD S/D se encuentra disponible en envases de 5 g y 10 g.
Cada envase contiene
- un vial de polvo de 10 g
- 192 ml de agua para preparaciones inyectables
- un dispositivo estéril de transferencia
- un equipo estéril de administración con filtro
Titular de la autorización de comercialización y responsable de la fabricación:
Titular de la autorización de comercialización:
Baxalta Innovations GmbH
Industriestrasse 67
1221 Viena
Austria
Responsable de la fabricación:
Baxalta Belgium Manufacturing SA
Boulevard René Branquart, 80 (Lessines)
B-7860-Bélgica
Representante local:
Takeda Farmacéutica España S.A.
Calle Albacete, 5, planta 9ª,
Edificio Los Cubos
28027 Madrid
España
Tel: +34 91 790 42 22
Este prospecto ha sido aprobado en Enero 2021.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
---------------------------------------------------------------------------------------------------------------------------
ESTA INFORMACIÓN ESTÁ DESTINADA ÚNICAMENTE A MÉDICOS O PROFESIONALES DEL SECTOR SANITARIO
Precauciones especiales durante la conservación
Se ha demostrado una estabilidad en uso química y física de GAMMAGARD S/D reconstituido de 24 horas a temperatura ambiente. Desde el punto de vista microbiológico el producto debe utilizarse inmediatamente, el tiempo y las condiciones de conservación antes del uso son responsabilidad del usuario y normalmente no deberían ser superiores a 24 horas a 2-8ºC, cuando la reconstitución se haya realizado en condiciones asépticas controladas y validadas.
Reconstitución: Usar una técnica aséptica:
Después de la reconstitución sólo se administrarán soluciones transparentes o ligeramente opalescentes e incoloras o amarillentas.
Llevar el vial de polvo y el vial de agua para preparaciones inyectables (disolvente) a temperatura ambiente. Mantener esta temperatura hasta completar la disolución.
- Solución al 5%
- Quitar los protectores de los viales y limpiar los tapones con solución germicida.
- Quitar el protector que cubre el punzón del dispositivo de transferencia. No tocar el punzón.
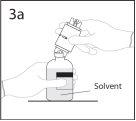 3a. Colocar el vial de disolvente sobre una superficie lisa.
3a. Colocar el vial de disolvente sobre una superficie lisa.
Utilizar el extremo del punzón que queda al descubierto para
perforar el vial de disolvente a través del centro del tapón.
PRECAUCIÓN: si no se introduce el punzón en el centro del
tapón, éste puede soltarse y perderse el vacío.
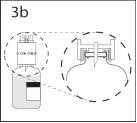 3b. Asegurar que el cuello del vial queda encajado totalmente en
3b. Asegurar que el cuello del vial queda encajado totalmente en
El dispositivo presionando firmemente el dispositivo de transferencia.
Quitar el protector que cubre el otro extremo del punzón mientras
se sujeta el dispositivo de transferencia. No tocar el punzón.
- Mantener el vial de disolvente con el dispositivo de transferencia conectado en un ángulo con respecto al vial de polvo para prevenir que el disolvente se salga.
Nota: no colocar el vial de disolvente hacia abajo ya que el disolvente se puede derramar.
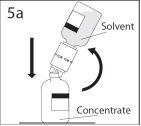 5a. Perforar el vial de polvo a través del centro del
5a. Perforar el vial de polvo a través del centro del
tapón mientras que se invierte rápidamente el vial de disolvente
para evitar que el disolvente se salga.
PRECAUCIÓN: si no se introduce el punzón en el
centro del tapón, éste puede soltarse y perderse el
vacío.
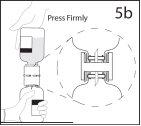 5b. Asegurar que el cuello del vial queda encajado totalmente
5b. Asegurar que el cuello del vial queda encajado totalmente
en el dispositivo presionando firmemente el vial de disolvente.
- Después de que todo el disolvente haya pasado al
vial de polvo, retirar el dispositivo de transferencia y el vial
de disolvente vacío. Girar inmediatamente el vial de concentrado para mezclar totalmente el contenido.
PRECAUCIÓN: no agitar. Evitar la formación de espuma.
Después de un único uso, desechar el dispositivo de transferencia.
- Solución al 10%
- Quitar los protectores de los viales y limpiar los tapones con solución germicida.
- Para preparar una solución al 10% es necesario extraer la mitad del volumen del disolvente. La tabla 2 describe el volumen de disolvente que se debe extraer de cada vial para conseguir una solución al 10% antes de conectar el dispositivo de transferencia. Usando una técnica aséptica, retirar el volumen no necesario de disolvente utilizando una jeringa hipodérmica estéril y una aguja. Desechar la jeringa y la aguja que contienen la cantidad de disolvente no requerida.
- Utilizando el disolvente residual en el vial del disolvente, seguir los pasos 2-6 descritos en la sección A.
TABLA 2
10 g
Concentración vial
5% Para la reconstitución al 5% no extraer ninguna cantidad de disolvente
10% 96 ml
Administración. Usar una técnica aséptica
Seguir las instrucciones de uso del folleto que acompaña al equipo de administración incluido en el envase. Si se utiliza otro equipo de administración, asegurarse que contiene un filtro similar.
Instrucciones de uso y manipulación
El producto debe llevarse a temperatura ambiente o temperatura corporal antes de su uso.
La disolución completa debe conseguirse en 30 minutos.
La solución resultante debe ser transparente o ligeramente opalescente e incolora o amarillenta. No utilizar soluciones que estén turbias o contengan sedimentos. El producto reconstituido debe inspeccionarse visualmente antes de su administración para verificar la ausencia de partículas y coloración.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con las normativa local.
Eliminar el equipo de transferencia después de su único uso.
Forma de administración
Vía intravenosa.
Si es posible, se recomienda que la solución al 10% de GAMMAGARD S/D se administre a través de las venas antecubitales. Esto puede reducir la probabilidad de que se produzcan molestias en el lugar de la perfusión.
GAMMAGARD S/D al 5% (50 mg/ml) debe administrarse por vía intravenosa a una velocidad inicial de 0,5 ml/kg/h. En general, se recomienda que los pacientes que reciban GAMMAGARD S/D por primera vez o que cambien de otra inmunoglobulina intravenosa a GAMMAGARD S/D, inicien el tratamiento con la velocidad de administración más baja y luego aumenten a la velocidad máxima, si previamente han tolerado varias perfusiones a velocidades de perfusión intermedias.
Si se tolera bien, la velocidad de administración de la solución al 5% puede aumentarse gradualmente hasta un máximo de 4 ml/kg/h. Cuando se cambie de una solución al 5% a una solución al 10%, la velocidad de administración de la solución al 10% debe ser inicialmente baja para mantener comparable la velocidad de administración de la proteína IgG. En muchos pacientes es posible aumentar gradualmente la velocidad de administración de la solución al 10% hasta 8 ml/kg/h. La velocidad de administración se ajustará individualmente de acuerdo a la tolerabilidad del paciente.
Precauciones especiales
Cualquier efecto adverso relacionado con la perfusión debe ser tratado reduciendo la velocidad o parando la perfusión.
Cada vez que se administra GAMMAGARD S/D se recomienda indicar el nombre y el número de lote del producto.
Incompatibilidades
GAMMAGARD S/D no debe mezclarse con otros medicamentos. Se recomienda administrar GAMMAGARD S/D de forma separada a otros medicamentos que reciba el paciente.
Dosificación recomendada
INDICACIÓN | DOSIS | FRECUENCIA DE INYECCIÓN/PERFUSIÓN |
Tratamiento de reposición en inmunodeficiencia primaria Tratamiento de reposición en inmunodeficiencia secundaria SIDA congénito Hipogammaglobulinemia(< 4 g/l) en pacientes que han recibido un trasplante alogénico de células madre hematopoyéticas | Dosis inicial: 0,4 – 0,8 g/kg Continuación: 0,2-0,8 g/kg 0,2-0,4 g/kg 0,2-0,4 g/kg 0,2-0,4 g/kg | cada 3-4 semanas para obtener un nivel valle de IgG de, al menos, 5-6 g/l. cada 3-4 semanas para obtener un nivel valle de IgG de, al menos, 5-6 g/l. cada 3-4 semanas cada 3-4 semanas para obtener un nivel valle de IgG por encima de 5 g/l |
Inmunomodulación: Trombocitopenia inmune primaria Síndrome de Guillain-Barré Enfermedad de Kawasaki | 0,8-1 g/kg o 0,4 g/kg /día 0,4 g/kg /día 1,6-2 g/kg o 2 g/kg | el 1er día, pudiéndose repetir una vez dentro de los tres días siguientes durante 2-5 días durante 5 días en varias dosis durante 2-5 días, junto con ácido acetilsalicílico en una dosis, junto con ácido acetilsalicílico. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GAMMAGARD S/D 10 G, POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 100 mg/mlPrincipio activo: immunoglobulins, normal human, for intravascular adm.Fabricante: Instituto Grifols S.A.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 mg/mlPrincipio activo: immunoglobulins, normal human, for intravascular adm.Fabricante: Instituto Grifols S.A.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 mg/mlPrincipio activo: immunoglobulins, normal human, for intravascular adm.Fabricante: Instituto Grifols S.A.Requiere receta
Médicos online para GAMMAGARD S/D 10 G, POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GAMMAGARD S/D 10 G, POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















