
FRIDEX 1 MG/ML COLIRIO EN SOLUCION

Cómo usar FRIDEX 1 MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Fridex 1 mg/ml colirio en solución
dexametasona fosfato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fridex y para qué se utiliza
- Qué necesita saber antes de empezar a usar Fridex
- Cómo usar Fridex
- Posibles efectos adversos
- Conservación de Fridex
- Contenido del envase e información adicional
1. Qué es Fridex y para qué se utiliza
Este medicamento contiene el principio activo dexametasona, que es un corticoide indicado para el tratamiento de los síntomas inflamatorios (como dolor, calor, hinchazón y enrojecimiento).
Este medicamento está indicado para el tratamiento de la inflamación de sus ojos.
Si tiene infección en un ojo (ojo rojo, lágrimas y mucosidad), se le administrará otro medicamento para usarlo al mismo tiempo que Fridex (ver sección 2).
2. Qué necesita saber antes de empezar a usar Fridex
No use Fridex:
- Si es alérgico a dexametasona fosfato o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- Si tiene una infección en el ojo que puede ser causada por una bacteria (infección purulenta aguda), por hongos, por virus (virus del herpes, virus vaccinia, virus de la varicela) o por amebas (un parásito);
- Si tiene daños en la superficie del ojo (pequeñas perforaciones, úlceras o lesiones que no se han curado correctamente);
- Si tiene la presión ocular alta y sabe que es causada por glucocorticosteroides (familia de los medicamentos corticosteroides)
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Fridex.
No inyectar, no ingerir este medicamento.
Evite el contacto de la punta del envase dispensador con el ojo o los párpados.
- Se necesita un estricto control del ojo durante el uso de Fridex en particular:
- Para niños y personas de edad avanzada, se recomienda un control más frecuente.
- Si tiene una infección ocular. Utilice este medicamento sólo si está siendo tratado al mismo tiempo con un medicamento antiinfeccioso.
- Si tiene una úlcera corneal (una llaga abierta en la superficie del ojo con dolor a veces extremo, lágrimas, ojos entrecerrados y pérdida de visión) no utilice este medicamento, a menos que la inflamación sea la causa del retraso en la curación.
- Si sufre de presión ocular alta. Si ya ha tenido presión ocular alta después de un tratamiento esteroide ocular, corre el riesgo de tenerlo nuevamente si usa este medicamento.
- Si tiene glaucoma, una enfermedad que puede causar daño al nervio óptico y puede causar pérdida de visión.
- Niños: no usar durante un tratamiento a largo plazo sin descanso.
- Si tiene conjuntivitis alérgica grave (enrojecimiento, hinchazón, picazón y lágrimas en los ojos) que otro medicamento no ha podido tratar. Use este medicamento solo durante un corto período de tiempo.
- Si es diabético, informe a su oftalmólogo u óptico sobre su enfermedad antes de usar este medicamento.
- Si tiene un ojo rojo que no ha sido diagnosticado, no use Fridex sin antes consultar con su médico.
- Evite llevar lentes de contacto durante el uso de este medicamento.
Consulte a su médico si nota hinchazón y aumento de peso alrededor del tronco y en la cara, ya que estos son generalmente los primeros signos de una afección llamada síndrome de Cushing. Se puede desarrollar una disminución de la función de la glándula suprarrenal tras interrumpir un tratamiento a largo plazo o intensivo con este medicamento. Consulte a su médico antes de interrumpir el tratamiento por su cuenta. Estos riesgos son especialmente importantes en niños y pacientes tratados con un medicamento llamado ritonavir o cobicistat (usados en el tratamiento del VIH).
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Otros medicamentos y Fridex
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Si está usando otros colirios, espere 15 minutos para instilar el otro medicamento.
El uso conjunto de colirios que contienen esteroides y colirios que contienen betabloqueantes (para tratar la presión alta en el ojo) puede causar la sedimentación de fosfato de calcio en la superficie del ojo.
Informe a su médico si está utilizando ritonavir o cobicistat (usados en el tratamiento del VIH), ya que estos medicamentos pueden aumentar la cantidad de dexametasona en sangre.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No hay suficiente información sobre el uso de este medicamento durante el embarazo para evaluar los posibles efectos adversos. Por esta razón, no se recomienda el uso de este medicamento durante el embarazo.
No se sabe si este medicamento pasa a la leche materna. Sin embargo, la dosis de este medicamento es baja, por lo tanto, este medicamento se puede usar durante la lactancia si su médico lo considera apropiado y bajo supervisión médica.
Conducción y uso de máquinas:
Es posible que tenga visión borrosa durante un corto periodo de tiempo después de usar este colirio. Espere hasta que su visión sea normal antes de conducir o usar máquinas.
Fridex contiene fosfatos
Este medicamento contiene 13,385 mg de fosfatos en cada frasco que equivale a 2,677 mg/ml. Si sufre de daño severo en la córnea (la capa transparente de la parte frontal del ojo) el tratamiento con fosfatos, en casos muy raros, puede provocar visión borrosa por acumulación de calcio.
3. Cómo usar Fridex
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Dosis recomendada
La dosis habitual recomendada es 1 gota, de 4 a 6 veces al día en el ojo a tratar.
En casos graves, el tratamiento puede iniciarse con 1 gota cada hora y debe reducirse a 1 gota cada 4 horas cuando se observe una respuesta favorable. Se recomienda la suspensión gradual del tratamiento con el fin de evitar una recaída.
Uso en personas de edad avanzada
No se necesita ajustar la dosis del medicamento.
Uso en niños
No utilice este medicamento en niños durante un tratamiento a largo plazo sin descanso.
Forma de administración
Este medicamento debe ser instilado en el ojo. No inyecte ni trague el medicamento.
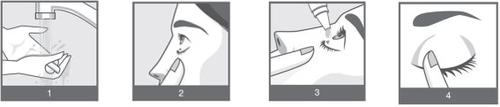
- Lávese bien las manos antes de su uso (Figura 1).
- Retire la tapa.
- Sostenga el frasco, apuntando hacia abajo, con el pulgar y los dedos.
- Mire hacia arriba y tire suavemente con su dedo del párpado inferior hacia abajo hasta que se forme una “bolsa” entre el párpado y el ojo (Figura 2).
- Incline la cabeza hacia atrás y coloque una gota en el ojo a tratar (Figura 3).
- No toque con el gotero el ojo o el párpado, las áreas circundantes u otras superficies. Podría infectar el colirio.
- Inmediatamente después de colocar la gota en los ojos, presione ligeramente con el dedo el lagrimal (el borde del ojo que se junta con la nariz) durante unos minutos. Esto ayuda a que el medicamento no salga del ojo. (Figura 4).
- Cierre firmemente la tapa del frasco inmediatamente después de su uso.
Duración del tratamiento
Necesitará usar sus gotas durante varios días. No use este medicamento durante más de 14 días.
Si usa más Fridex del que debe
Si se ha puesto demasiado medicamento en su ojo y nota sensación de irritación duradera, enjuague el ojo con agua estéril.
Informe a su médico o farmacéutico inmediatamente.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida
Si olvidó usar Fridex
No use una dosis doble para compensar la dosis olvidada.
Si interrumpe el tratamiento con Fridex
No deje de usar este medicamento bruscamente. Informe a su médico si está pensando en suspender el tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- Presión alta en el ojo después de 2 semanas de usar las gotas.
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Molestias en el ojo, irritación, ardor, escozor, picazón y visión borrosa después de su uso. Estos suelen ser leves y no duran mucho.
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- Signos de una reacción alérgica
- Curación de heridas que tarda más de lo esperado
- Lente turbia (catarata)
- Infecciones oculares
- Presión alta en el ojo (glaucoma)
- Si se usa con frecuencia, es posible que los riñones no produzcan suficientes hormonas (supresión de la función corticoadrenal). Esto podría provocar niveles bajos de azúcar en la sangre, deshidratación, pérdida de peso y desorientación.
Muy raras: pueden afectar hasta 1 de cada 10.000 personas.
- Inflamación en la superficie del ojo que produce ojos rojos, lágrimas e irritación (conjuntivitis)
- Pupila dilatada (midriasis)
- Hinchazón de la cara (edema facial)
- Párpados caídos (ptotis)
- Inflamación del ojo que causa dolor y enrojecimiento (uveítis)
- Sedimento de calcio en la superficie del ojo (calcificación de la córnea)
- Superficie inflamada del ojo que proporciona visión borrosa, ojos secos, sensibilidad a la luz, ardor, lágrimas y una sensación arenosa en el ojo (queratopatía cristalina)
- Cambios en el grosor de la superficie del ojo
- Hinchazón en la superficie del ojo (edema corneal)
- Ulceración de la superficie del ojo que causa dolor, lágrimas, ojos entrecerrados y pérdida de visión
- Pequeñas perforaciones en la superficie del ojo (perforación de la córnea)
Frecuencia no conocida: no puede estimarse a partir de los datos disponibles.
Problemas hormonales: crecimiento excesivo de vello corporal (particularmente en las mujeres), debilidad y desgaste muscular, estrías moradas en la piel, aumento de la presión arterial, menstruaciones irregulares o ausentes, cambios en los niveles de proteínas y calcio del cuerpo, retraso en el crecimiento en niños y adolescentes e hinchazón y aumento de peso del cuerpo y la cara (llamado “Síndrome de Cushing”) (ver sección 2, “Advertencias y precauciones”).
En muy raras ocasiones algunas personas con un daño grave en la capa trasparente de la parte frontal del ojo (córnea) han desarrollado parches nublados en la córnea debido a la acumulación del calcio durante el tratamiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fridex
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
No utilice el colirio pasados 28 días tras la primera apertura del frasco.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Fridex
- El principio activo es dexametasona fosfato.
- Cada ml de solución contiene 1 mg de dexametasona fosfato (como dexametasona fosfato sódico).
Los demás componentes son: edetato de disodio, hidrogenofosfato de disodio anhidro, cloruro de sodio y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Es una solución transparente, incolora de 5 ml sin partículas visibles en un frasco blanco LDPE de 5 ml con boquilla Novelia blanca (HDPE y silicona) y tapón de HDPE blanco.
Titular de la autorización de comercialización
NTC S.r.l.
Via Luigi Razza, 3
20124
Milán - Italia
Responsable de la fabricación
Rafarm S.A.
Thesi Pousi-Xatzi Agiou Louka
Paiania Attiki 19002, P.O.Box37
Grecia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
NTC Ophthalmics Iberica, S.L.
Calle Pinar, 5
28006 Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Italia: Fridex
España: Fridex
Fecha de la última revisión de este prospecto:Junio 2020
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)
http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FRIDEX 1 MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 1 mg/mlPrincipio activo: DexametasonaFabricante: Pharmaselect International Beteiligungs GmbhRequiere recetaForma farmacéutica: COLIRIO, 0,1 %Principio activo: DexametasonaFabricante: Fidia Farmaceutici S.P.A.Requiere recetaForma farmacéutica: COLIRIO, 1mg/mlPrincipio activo: DexametasonaFabricante: Laboratoires TheaRequiere receta
Médicos online para FRIDEX 1 MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FRIDEX 1 MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















