
FENILEFRINA ALTAN 0,1 MG/ML SOLUCION INYECTABLE Y PARA PERFUSION

Cómo usar FENILEFRINA ALTAN 0,1 MG/ML SOLUCION INYECTABLE Y PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Fenilefrina Altan 0,1 mg/ml solución inyectable y para perfusión
Fenilefrina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fenilefrina Altan 0.1 mg/ml y para qué se utiliza
- Qué necesita saber antes de empezar a usar Fenilefrina Altan 0.1 mg/ml
- Cómo usar Fenilefrina Altan 0.1 mg/ml
- Posibles efectos adversos
- Conservación de Fenilefrina Altan 0.1 mg/ml
- Contenido del envase e información adicional.
1. Qué es Fenilefrina Altan 0.1 mg/ml y para qué se utiliza
Este medicamento pertenece al grupo llamado agentes adrenérgicos o dopaminérgicos.
Fenilefrina Altan 0.1 mg/ml se usa para tratar la presión arterial baja (hipotensión) que puede ocurrir durante los diferentes tipos de anestesia.
2. Qué necesita saber antes de empezar a usar Fenilefrina Altan 0.1 mg/ml
No deben administrarle Fenilefrina Altan 0.1 mg/ml
- Si es alérgico (hipersensible) a la fenilefrina o cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si está siendo tratado con un medicamento conocido como inhibidor de la monoaminooxidasa o ha interrumpido el tratamiento con este tipo de medicamento en los últimos 14 días.
- Si tiene presión arterial alta.
- Si padece una enfermedad vascular periférica (mala circulación sanguínea).
- Si tiene actividad excesiva de la glándula tiroides.
Advertencias y precauciones
Se requiere precaución al administrar Fenilefrina Altan 0.08 mg/ml en pacientes con:
- enfermedad cardiovascular preexistente
- diabetes mellitus
- hipertensión arterial
- enfermedad isquémica del corazón
- arritmia
- bradicardia
- bloqueo cardíaco incompleto
- taquicardia
- enfermedad vascular periférica oclusiva, incluida la arteriosclerosis
- aneurisma
- en pacientes con angina de pecho, la fenilefrina puede precipitar o exacerbar la angina.
- glaucoma de ángulo cerrado.
La fenilefrina puede inducir una reducción en el gasto cardíaco. Por lo tanto, se requiere precaución cuando se administra a pacientes con aterosclerosis, ancianos y pacientes con circulación cerebral o coronaria comprometida.
En pacientes con insuficiencia cardíaca grave o shock cardiogénico, la fenilefrina puede empeorar la insuficiencia cardíaca como consecuencia de la vasoconstricción inducida y el aumento de la poscarga.
Se debe prestar especial atención a la inyección de fenilefrina para prevenir la extravasación, ya que esto puede causar necrosis tisular.
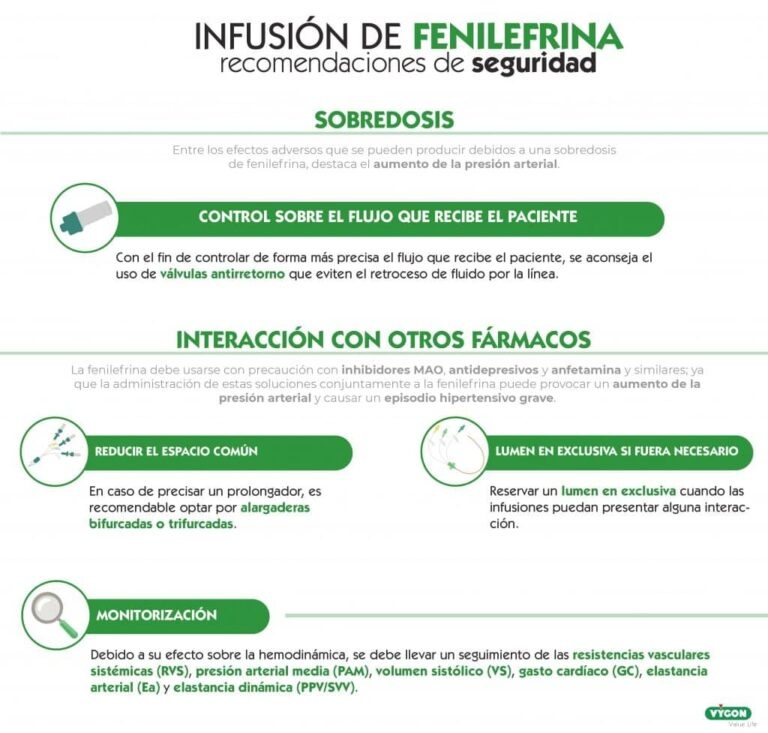
Uso de Fenilefrina Altan 0.1 mg/ml con otros medicamentos
Informe a su médico o enfermero si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Fenilefrina Altan 0.1 mg/ml puede interactuar con los siguientes medicamentos:
Combinaciones contraindicadas (ver sección 4.3)
Inhibidores no selectivos de la monoaminooxidasa (MAO) (fenelzina, tranilcipromina)
Hipertensión paroxística, posiblemente hipertermia mortal. Debido a la larga duración de la acción inhibidora de la MAO, esta interacción aún es posible 15 días después de que se suspenda el inhibidor de la MAO.
Combinaciones no recomendables
Alcaloides dopaminergicos (bromocriptina, cabergolina, lisurida, pergolida):
Riesgo de vasoconstricción y / o crisis hipertensiva.
Alcaloides vasoconstrictores (dihidroergotamina, ergotamina, metilergometrina, metisergida):
Riesgo de vasoconstricción y / o crisis hipertensiva.
Antidepresivos tricíclicos (por ejemplo, imipramina):
Hipertensión paroxística con posibilidad de arritmia (inhibición de la entrada de adrenalina o noradrenalina en fibras simpáticas).
Antidepresivos noradrenérgicos-serotoninérgicos (milnacipran, venlafaxina):
Hipertensión paroxística con posibilidad de arritmia (inhibición de la entrada de adrenalina o noradrenalina en fibras simpáticas).
Inhibidores selectivos de la monoaminooxidasa (MAO) tipo A (moclobemida, toloxatona)
Riesgo de vasoconstricción y/o episodios de hipertensión.
Linezolid:
Riesgo de vasoconstricción y/o crisis hipertensiva.
Guanetidina y productos relacionados:
Aumento sustancial de la presión arterial (hiperreactividad relacionada con la reducción del tono simpático y / o la inhibición de la entrada de adrenalina o noradrenalina en las fibras simpáticas). Si no se puede evitar la combinación, use con precaución dosis más bajas de agentes simpaticomiméticos.
Glucósidos cardíacos, quinidina:
Mayor riesgo de arritmias.
Sibutramina
Hipertensión paroxística con posibilidad de arritmias (inhibición de la entrada de adrenalina o noradrenalina en fibras simpáticas).
Anestésicos volátiles halogenados (desflurano, enflurano, halotano, isoflurano, metoxiflurano, sevoflurano):
Riesgo de crisis hipertensivas y arritmia perioperatoria.
Combinaciones que requieren precauciones de uso:
Antihipertensivos, incluidos los bloqueadores de los receptores α y β.
La fenilefrina puede elevar la presión arterial y, por lo tanto, revertir la acción de muchos agentes antihipertensivos. La interacción entre fenilefrina y bloqueantes de los receptores α y β puede ser compleja. Los medicamentos que actúan sobre los adrenoreceptores alfa-1 pueden potenciar la acción de la fenilefrina (como granisetrón) o disminuir (como doxazosina o buspiron).
Agentes oxitocicos:
El efecto de las aminas simpaticomiméticas presoactivas se potencia. Por lo tanto, algunos agentes oxitócicos pueden causar hipertensión persistente grave y pueden ocurrir accidentes cerebrovasculares durante el período posparto.
Niños
No se recomienda el uso de este medicamento en niños debido a datos insuficientes sobre eficacia, seguridad y recomendaciones de dosificación.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico, farmacéutico o enfermero antes de tomar este medicamento.
Embarazo
Cuando se administra en las últimas etapas del embarazo o durante el parto, Fenilefrina Altan 0.1 mg/ml puede dañar al feto. Es posible usar fenilefrina inyectable durante el embarazo de acuerdo con las indicaciones.
Lactancia
La cantidad de clorhidrato de fenilefrina que pasa a la leche materna parece ser pequeña.
Conducción y uso de máquinas
No hay información sobre cómo Fenilefrina Altan 0.1 mg/ml afecta su capacidad para conducir y utilizar máquinas.
Fenilefrina Altan 0,1 mg/ml contiene sodio
Este medicamento contiene 366,2 mg mg de sodio (componente principal de la sal de mesa/para
cocinar) en cada 100 ml. Esto equivale al 18,3% de la ingesta diaria máxima de sodio recomendada para un adulto.
3. Cómo usar Fenilefrina Altan 0.1 mg/ml
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico..
Fenilefrina Altan 0,1 mg/ml siempre debe ser administrado por un profesional de la salud y nunca por el paciente (ver sección 6).
La dosis recomendada es:
- Inyección en bolo intravenoso:
La dosis normal es de 50 a 100 microgramos, que puede repetirse hasta obtener el efecto deseado. Una dosis de bolo no debe exceder los 100 microgramos.
- Perfusión continua:
La dosis inicial es de 25 a 50 microgramos/min. Las dosis pueden aumentarse o disminuirse para mantener la presión sanguínea sistólica cerca del valor normal. Se ha evaluado que las dosis entre 25 y 100 microgramos/min son efectivas.
Población pediatrica
No se ha establecido la seguridad y eficacia de la fenilefrina en niños menores de 18 años. No hay datos disponibles.
Insuficiencia renal
Es posible que se necesiten dosis más bajas de fenilefrina en pacientes con insuficiencia renal.
Insuficiencia hepática
Es posible que se necesiten dosis más altas de fenilefrina en pacientes con cirrosis hepática.
Edad avanzada
El tratamiento en personas de edad avanzada se debe realizar con cuidado..
Si usa más Fenilefrina Altan 0.1 mg/ml del que debe
Los síntomas de sobredosis incluyen dolor de cabeza, náuseas, vómitos, psicosis paranoide, alucinaciones, aumento de la presión arterial, bradicardia refleja y ritmo cardíaco irregular. En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
El tratamiento debe consistir en medidas sintomáticas y de apoyo. Los efectos hipertensivos (presión arterial elevada) pueden tratarse con medicamentos conocidos como bloqueadores α- adrenérgicos, como la fentolamina, 5-60 mg por vía intravenosa durante 10-30 minutos, repetidos según sea necesario.
Si olvidó usarFenilefrina Altan 0.1 mg/ml
No use una dosis doble para compensar una dosis olvidada. Si tiene alguna duda sobre el uso de este medicamento, consulte a su médico o farmacéutico.
Fenilefrina Altan 0.1 mg/ml contiene sodio
Este medicamento contiene 366.2 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada 100 ml. Esto equivale al 18.3% de la ingesta diaria máxima de sodio recomendada para un adulto.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos secundarios se pueden clasificar según la frecuencia de la siguiente manera: Muy frecuentes: (pueden afectar a más de 1 de cada 10 personas), Frecuentes (pueden afectar hasta 1 de cada 10 personas), Poco frecuentes (pueden afectar hasta 1 de cada 100 personas), Raras (pueden afectar hasta 1 de cada 1.000 personas), Muy raras (pueden afectar hasta 1 de cada 10.000 personas) y Desconocidas (la frecuencia no se puede estimar con los datos disponibles)
Los efectos adversos más comunes de la fenilefrina son bradicardia, episodios hipertensivos, náuseas y vómitos. La hipertensión es más frecuente con dosis altas.
El efecto adverso cardiovascular más comúnmente reportado parece ser la bradicardia, probablemente debido a la estimulación vagal mediada por barorreceptores y consistente con el efecto farmacológico de la fenilefrina.
Se han informado las siguientes reacciones adversas durante el uso de fenilefrina, aunque su frecuencia no se ha establecido claramente:
Trastornos del sistema inmunitario:
hipersensibilidad
Trastornos psiquiátricos:
ansiedad, excitabilidad, agitación, estados psicóticos, confusión.
Trastornos del sistema nervioso:
dolor de cabeza, hemorragia cerebral, vértigo, desmayo, letargo (inactividad mental o física), insomnio, parestesia.(sensación dérmica anormal), temblor (temblor involuntario del cuerpo o las extremidades).
Trastornos oculares:
midriasis, agravamiento del glaucoma de ángulo cerrado preexistente.
Trastornos cardíacos:
bradicardia refleja (ritmo cardíaco lento), taquicardia refleja(ritmo cardíaco rápido), arritmia cardíaca (ritmo cardíaco irregular), dolor anginoso, palpitaciones, paro cardíaco.
Trastornos vasculares
hipertensión (presión arterial alta), hipotensión (presión arterial baja), rubor (enrojecimiento), crisis hipertensiva.
Trastornos respiratorios, torácicos y mediastínicos:
disnea (dificultades respiratorias), edema pulmonar (inflamación del pulmón).
Trastornos gastrointestinales:
náuseas, vómitos, hipersalivación.
Trastornos de la piel y del tejido subcutáneo:
sudoración, hormigueo temporal, enfriamiento de la piel, palidez o palidez de la piel, piloerección
Trastornos renales y urinarios:
dificultad para orinar, retención urinaria.
Trastornos del metabolismo y de la nutrición:
alteraciones del metabolismo de la glucosa.
Trastornos generales y alteraciones en el lugar de administración:
la extravasación de fenilefrina puede causar necrosis tisular (muerte tisular).
Si tiene algún efecto adverso, hable con su médico o farmacéutico. Esto incluye los posibles efectos adversos que no figuran en este prospecto.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fenilefrina Altan 0.1 mg/ml
Mantener este medicamento fuera de la vista y del alcance de los niños.
Este medicamento no requiere ninguna temperatura especial de conservacion para bolsas de polipropileno.
No conservar a temperatura superior a 30ºC para bolsas de poliolefina sin PVC.
Conservar en el embalaje original para protegerlo de la luz.
No utilize este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No congelar
Su médico almacenará este medicamento por usted.
Desde un punto de vista microbiológico, el producto debe usarse inmediatamente después de la primera apertura.
Fenilefrina Altan 0.1 mg/ml no debe usarse si hay partículas visibles en él.
Los medicamentos no se deben tirar por los desagües. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Fenilefrina Altan 0,1 mg/ml
- El principio activo es fenilefrina hidrocloruro. Cada bolsa contiene 10 mg de fenilefrina hidrocloruro que equivalen a 8 mg de fenilefrina base.
- Los demás componentes son cloruro de sodio, citrato de sodio, ácido cítrico y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Fenilefrina Altan 0,1 mg/ml es una solución transparente e incolora. Cada bolsa de polipropileno o de poliolefina PVC libre contiene 100 ml de solución.
Tamaños de envases
Bolsa de polipropileno: 10 x 100 ml
Bolsa de poliolefina PVC-libre: 10 x 100 ml
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Altan Pharmaceuticals S.A.
C/ Cólquide, Nº 6, Portal 2, 1ª Planta, Oficina F.
Edificio Prisma, Las Rozas, 28230 Madrid – España
Responsable de la fabricación
Altan Pharmaceuticals, S.A.
Polígono Industrial de Bernedo, s/n,
01118 Bernedo, Álava.- España
Fecha de la última revisión de este prospecto:enero 2021
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos sanitarios (AEMPS) http://www.aemps.gob.es
----------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Fenilefrina Altan 0,1 mg/ml solución inyectable y para perfusión
Posología y forma de administración:
Administración parenteral o perfusión intravenosa. Fenilefrina Altan 0,1 mg/ml solución inyectable y para perfusión solo debe ser administrada por profesionales de la salud con formación y experiencia adecuada.
1.Inyección en bolo intravenoso:
La dosis normal es de 50 a 100 microgramos., que puede repetirse hasta alcanzar el efecto deseado. Una dosis de bolo no debe exceder los 100 microgramos.
- Perfusión continua:
La dosis inicial es de 25 a 50 microgramos/min. Las dosis pueden aumentarse o disminuirse para mantener la presión sanguínea sistólica cerca del valor normal. Se ha evaluado que las dosis entre 25 y 100 microgramos/min son efectivas.
Pacientes de edad avanzada:
El tratamiento de pacientes de edad avanzada debe llevarse a cabo con cuidado.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FENILEFRINA ALTAN 0,1 MG/ML SOLUCION INYECTABLE Y PARA PERFUSIONForma farmacéutica: INYECTABLE, 100 microgramos/mlPrincipio activo: fenilefrinaFabricante: Laboratoire AguettantRequiere recetaForma farmacéutica: INYECTABLE, 50 microgramos/mlPrincipio activo: fenilefrinaFabricante: Laboratoire AguettantRequiere recetaForma farmacéutica: INYECTABLE, 10 MG/MLPrincipio activo: fenilefrinaFabricante: Altan Pharmaceuticals SaRequiere receta
Médicos online para FENILEFRINA ALTAN 0,1 MG/ML SOLUCION INYECTABLE Y PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FENILEFRINA ALTAN 0,1 MG/ML SOLUCION INYECTABLE Y PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











