
DARAPRIM 25 mg COMPRIMIDOS


Cómo usar DARAPRIM 25 mg COMPRIMIDOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Daraprim 25 mg comprimidos
Pirimetamina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo. Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Daraprim y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Daraprim
- Cómo tomar Daraprim
- Posibles efectos adversos
- Conservación de Daraprim
- Contenido del envase e información adicional
1. Qué es Daraprim y para qué se utiliza
Daraprim contiene pirimetamina que pertenece al grupo de medicamentos denominados antipalúdicos. Está indicado en adultos y niños para el tratamiento de la malaria no complicada producida por cepas sensibles de Plasmodium falciparum.
Daraprim en combinación con una sulfamida está indicado para el tratamiento de toxoplasmosis congénita y adquirida.
La pirimetamina no debe usarse como monoterapia en el tratamiento de la malaria y la toxoplasmosis.
2. Qué necesita saber antes de empezar a tomar Daraprim
No tome Daraprim
- si es alérgico al principio activo o a alguno de los demás componentes de este medicamento incluidos en la sección 6.
Advertencias y precauciones
- si presenta un tipo de anemia llamado anemia megaloblástica o síntomas de malnutrición, ya que la pirimetamina puede intensificar la falta de folatos asociada a este tipo de anemia. De acuerdo con esto, estos individuos deberán recibir un suplemento de ácido folínico
- si presenta un historial de convulsiones; en estos pacientes deberían evitarse elevadas dosis iniciales de pirimetamina.
Cuando la pirimetamina se administre con una sulfonamida, se deben tener en cuenta las precauciones generales aplicables a las sulfonamidas, en cualquier caso debe asegurarse una ingesta adecuada de líquidos para minimizar el riesgo de cristaluria.
Uso de Daraprim con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Especialmente la pirimetamina puede modificar el efecto de los siguientes medicamentos: antibióticos como cotrimoxazol o trimetoprim, antipalúdicos como proguanilo, antivirales como zidovudina, o agentes citostáticos (como, por ejemplo, metotrexato). La administración concomitante con una combinación de trimetoprim/sulfamida puede desarrollar anemia megaloblástica.
La administración concomitante de lorazepam (benzodiazepina) y pirimetamina puede inducir un leve daño del hígado.
Se han asociado casos de desaparición total o parcial de las células que se encuentran en condiciones normales en la médula ósea (aplasia fatal de la médula ósea) con la administración del antibiótico daunorrubicina y de pirimetamina a individuos que sufren un tipo de cáncer de los glóbulos blancos que se conoce como leucemia mieloide aguda.
Las sales antiácidas y el agente antidiarreico caolín reduce la absorción de la pirimetamina.
La pirimetamina puede afectar a la eficacia o toxicidad de fármacos como el antimalárico o antipalúdico quinina o anticoagulantes como la warfarina si se administran concomitantemente.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Daraprim sólo está recomendado en terapia de combinación con sulfamida, durante el segundo y tercer trimestre de embarazo. En el primer trimestre se recomienda una terapia alternativa.
Se recomienda la administración concomitante de folinato cálcico si se administra Daraprim durante el embarazo.
Pirimetamina pasa a la leche materna por lo que no se recomienda su uso durante la lactancia, a menos que su médico lo estime conveniente.
Conducción y uso de máquinas
No se ha observado que Daraprim influya sobre la capacidad para conducir vehículos y utilizar máquinas.
Daraprim 25 mg comprimidos contiene lactosa y sodio
Este medicamento contiene lactosa. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Daraprim
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Recuerde tomar su medicamento.
Su médico le indicará si debe incrementar o reducir la dosis del medicamento durante el período de tratamiento, así como la duración del mismo.
Daraprim son comprimidos para administración por vía oral. Se deben tomar por la mañana o por la noche acompañados o no de alimentos. Los comprimidos deben tragarse sin masticar con ayuda de un poco de líquido (un vaso de agua).
Todos los pacientes que reciban pirimetamina deben recibir un suplemento de ácido folínico para reducir el riesgo de afectación de la médula ósea.
Tratamiento de la malaria:
Daraprim deberá administrarse en dosis única concomitantemente con sulfadiazina y otro antimalárico.
Adultos:
Una única dosis de dos - tres comprimidos de Daraprim (50 a 75 mg de pirimetamina), junto con 1.000 mg – 1.500 mg de sulfadiazina.
En general, se debe administrar una dosis superior a adultos de más de 60 kg de peso.
Uso en niños:
Pirimetamina se puede usar por vía oral con sulfadoxina/sulfadiazina en niños a partir de 2 meses de edad.
Se recomienda la siguiente posología en base al peso corporal:
- Niños de 5 a 10 kg: 12,5 mg de pirimetamina en dosis única.
- Niños de 11 a 20 kg: 25 mg de pirimetamina en dosis única.
- Niños de 21 a 30 kg: 37,5 mg de pirimetamina en dosis única.
- Niños de 31 a 45 kg: 50 mg de pirimetamina en dosis única.
- Niños de más de 45 kg: emplear la misma dosis que para adultos.
Uso en pacientes de edad avanzada:
No existe información sobre el efecto de Daraprim en los pacientes de edad avanzada. A las dosis recomendadas para la malaria, no es probable que pirimetamina tenga efectos adversos en las personas de edad avanzada.
Tratamiento de toxoplasmosis
Daraprim se debe administrar concomitantemente con sulfadiazina o clindamicina. El uso de una sulfonamida alternativa puede requerir un ajuste de dosis.
Adultos inmunocompetentes:
Pirimetamina: Una dosis inicial de cuatro comprimidos (100 mg de pirimetamina) seguida de una dosis de uno – dos comprimidos diarios (25 - 50 mg al día de pirimetamina).
Adultos inmunodeficientes:
Daraprim deberá administrarse concomitantemente con sulfadiazina o clindamicina
- < 60 kg: pirimetamina 200 mg vía oral como dosis de carga, continuar con 50 mg al día.
- ≥ 60 kg: pirimetamina 200 mg vía oral como dosis de carga, seguido de 75 mg al día.
Posteriormente se administrará la pauta de profilaxis secundaria.
Uso en niños
En el tratamiento de toxoplasmosis, se recomiendan las siguientes pautas posológicas:
- Niños de menos de 3 meses (toxoplasmosis congénita): para el tratamiento de toxoplasmosis congénita se recomienda que los recién nacidos reciban pirimetamina 2 mg/kg/día durante 2 días; 1 mg/kg/día durante 2-6 meses, y después 1 mg/kg/día 3 veces a la semana hasta completar 12 meses de tratamiento.
- Niños de 3 a 9 meses: 6,25 mg diarios de pirimetamina junto con sulfadiazina: 100 mg/kg de peso corporal (máximo 1 g) al día en cuatro dosis divididas.
- Niños de 10 meses a 2 años: 1 mg/kg peso corporal/día de pirimetamina junto con 150 mg/kg peso corporal de sulfadiazina (máximo 1,5 g) al día en cuatro dosis divididas.
- Niños de 3 a 6 años: una dosis de carga de pirimetamina 2 mg/kg de peso corporal (hasta un máximo de 50 mg) seguida de una dosis de 1 mg/kg/día (dosis máxima de 25 mg); junto con 150 mg/kg peso corporal de sulfadiazina (máximo 2 g) al día en cuatro dosis divididas.
- Niños mayores de 6 años: igual que para adultos.
En niños inmunodeprimidos no están definidas las pautas posológicas. Como guía general, referirse a las pautas indicadas en niños con infecciones de toxoplasmosis.
Uso en pacientes de edad avanzada:
No existe información sobre el efecto de Daraprim en pacientes de edad avanzada. En teoría, es posible que dichos pacientes bajo tratamiento para la toxoplasmosis puedan ser más susceptibles a la afectación de la médula ósea producida por déficit de folatos asociado a la administración diaria de Daraprim.
Uso durante el embarazo:
25 mg al día hasta el parto.
Si toma más Daraprim del que debe
Si ha tomado más Daraprim de lo que debe, consulte inmediatamente a su médico o farmacéutico. Los síntomas más frecuentes en caso de sobredosis agudas son: vómitos y convulsiones. También puede presentarse ataxia, temblor y depresión respiratoria.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Daraprim
No tome una dosis doble para compensar las dosis olvidadas. Tome la siguiente dosis cuando corresponda.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Síntomas a los que debe estar atento
Daño de la médula ósea(fallo de la médula ósea para la producción de células de la sangre)
Esto aumenta el riesgo de sangrado y disminuye su capacidad para combatir infecciones. Los síntomas incluyen:
- sangrado o hematomas inexplicables
- fiebre
- dolor de garganta
- úlceras en la boca
- extrema palidez o debilidad
Avise a su médico tan pronto como sea posible si tiene cualquiera de estos síntomas – ya sea por primera vez, o si estos empeoran.
Los efectos adversos observados se clasifican según su frecuencia de presentación en: muy frecuentes (pueden afectar a más de 1 de cada 10 personas), frecuentes (pueden afectar hasta 1 de cada 10 personas), poco frecuentes (pueden afectar hasta 1 de cada 100 personas), raros (pueden afectar hasta 1 de cada 1.000 personas), muy raros (pueden afectar hasta 1 de cada 10.000 personas):
Efectos adversos muy frecuentes
Pueden afectar a más de 1 de cada 10pacientes:
- cefaleas (dolor de cabeza)
- vómitos, náuseas, diarrea
- erupción cutánea
Efectos adversos muy frecuentes que pueden aparecer en análisis de sangre:
- anemia (disminución en el número de glóbulos rojos)
Efectos adversos frecuentes
Pueden afectar hasta 1 de cada 10pacientes:
- vértigos
Los efectos adversos frecuentes que pueden aparecer en los análisis de sangre son:
- leucopenia (disminución en el número de glóbulos blancos)
- trombocitopenia (disminución en el número de plaquetas)
Dosis terapéuticas diarias de pirimetamina han mostrado deprimir la hematopoyesis (formación de células de la sangre) entre el 25 – 50% de los pacientes. La probabilidad de inducir leucopenia, anemia o trombocitopenia se reduce por la administración concomitante de folinato cálcico.
Efectos adversos poco frecuentes
Pueden afectar hasta 1 de cada 100pacientes:
- fiebre
- pigmentación anormal de la piel
Efectos adversos muy raros
Pueden afectar hasta 1 de cada 10.000pacientes:
- cólicos
- convulsiones
Las convulsiones se han observado predominantemente en pacientes tratados por toxoplasmosis
- úlceras bucales
- neumonía con infiltración celular y eosinofílica
Se ha observado cuando se administra pirimetamina una vez a la semana en asociación con sulfadoxina
- dermatitis.
Los efectos adversos muy raros que pueden aparecer en los análisis de sangre son:
- pancitopenia (disminución en el número de todos los tipos de células de la sangre)
La pancitopenia, en respuesta a folatos, se ha observado en pacientes con una probable deficiencia de folatos pre-existente. Se han producido muertes en ausencia de tratamiento de folatos.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: http://www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Daraprim
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25ºC. Conservar protegido de la luz.
No utilice este medicamento después de la fecha de caducidad (CAD) que aparece en el embalaje. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Daraprim
- El principio activo es pirimetamina. Cada comprimido contiene 25 mg de pirimetamina.
- Los demás componentes (excipientes) son: lactosa monohidrato, almidón de maíz, almidón de maíz hidrolizado, docusato de sodio y estearato de magnesio.
Aspecto del producto y contenido del envase
Daraprim son comprimidos de color blanco, biconvexos, ranurados y grabados con un código identificativo. Se presenta en envases con 30 comprimidos.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
SmithKline Beecham Farma, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Responsable de la fabricación
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublín 24
Irlanda
Fecha de la última revisión de este prospecto:octubre 2020.
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
Daraprim viene acondicionado en blíster resistente a niños.
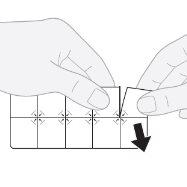
Cómo sacar un comprimido
- Separar un comprimido: tirar a lo largo de la línea precortada para separar un alvéolo del blíster.
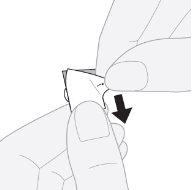
- Quitar la lámina externa: empezando por la esquina, levantar y quitar la lámina externa del alvéolo.
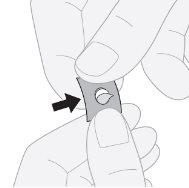
- Presione para sacar el comprimido: presione por un lado del comprimido para sacar el comprimido de la lámina de aluminio.
- País de registro
- Precio medio en farmacia4.9 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DARAPRIM 25 mg COMPRIMIDOSForma farmacéutica: INYECTABLE, 110 mgPrincipio activo: artesunateFabricante: Amivas Ireland LimitedRequiere recetaForma farmacéutica: COMPRIMIDO, 250 mg/100 mgPrincipio activo: proguanil and atovaquoneFabricante: Viatris LimitedRequiere recetaForma farmacéutica: COMPRIMIDO, 200 mgPrincipio activo: hydroxychloroquineFabricante: Products And Technology S.L.Requiere receta
Médicos online para DARAPRIM 25 mg COMPRIMIDOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DARAPRIM 25 mg COMPRIMIDOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















