
CLENSIA POWDER FOR ORAL SOLUTION

How to use CLENSIA POWDER FOR ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for the user
Clensia powder for oral solution
To consult the list of active ingredients, see section 6.
Read the entire leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Clensia and what is it used for
- What you need to know before taking Clensia
- How to take Clensia
- Possible side effects
- Storage of Clensia
- Package contents and additional information
1. What is Clensia and what is it used for
Clensia is an intestinal preparation with a lemon flavor available as an oral powder contained in 8 sachets.
There are 4 large sachets A and 4 small sachets B to be reconstituted in water before use.
You or your child is taking this medication to clean the intestine so that it is ready for examination.
Clensia works by emptying the contents of the intestine, so you or your child will feel bowel movements.
This medication is for use in adults, adolescents, and children from 6 years old.
2. What you need to know before taking Clensia
Do not take Clensia:
- if you or your child is allergic to macrogol or any of the other components of this medication (listed in section 6)
- if you or your child has a obstruction in the gastrointestinal tract
- if you or your child has a perforation in the gastrointestinal tract
- if you or your child has an alteration in gastric emptying
- if you or your child has intestinal paralysis (sometimes occurs after abdominal surgery)
- if you or your child has toxic colitis or toxic megacolon (a serious complication of acute colitis).
Clensia should not be administered to patients who are unconscious.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Clensia.
If you or your child has delicate health or a serious medical condition, you should be aware of the possible side effects indicated in section 4. If you are concerned, talk to your doctor or pharmacist.
You should consult your doctor before taking Clensia if you or your child has any of the following situations:
- you or your child needs consistent liquids to swallow safely
- tendency to regurgitate ingested beverages, food, or acid from the stomach
- kidney disease
- heart failure or heart disease, including high blood pressure and irregular heartbeats.
- dehydration (loss of body fluids that can lead to weight loss, thirst, dizziness, dry mouth, headache, dark and concentrated urine)
- acute flare-up of inflammatory bowel disease (Crohn's disease or ulcerative colitis)
Clensia should not be administered to patients with altered consciousness without medical supervision.
If you or your child experiences sudden abdominal pain or rectal bleeding while taking Clensia for intestinal preparation, contact your doctor or go to a doctor immediately.
Children
Do not give this medication to children from birth to 2 years old because the safety and efficacy of Clensia have not been established in these children. No data are available.
Do not give this medication to children between 2 and less than 6 years old because the efficacy of Clensia has not been established in these children. No recommendation can be made on the dosage based on the available data.Taking Clensia with other medications
If you or your child is taking other medications, take them at least one hour before taking Clensia or at least one hour after taking it, as they may be eliminated from the digestive tract and not work properly. In particular, a transient increase in blood pressure related to insufficient absorption of antihypertensive medications has been observed.
Inform your doctor or pharmacist if you or your child is taking, has recently taken, or may need to take any other medication.
If you or your child is taking medications that affect kidney function (such as diuretics, non-steroidal anti-inflammatory drugs, ACE inhibitors, and angiotensin receptor blockers), you have a higher risk of suffering from electrolyte disturbances when using Clensia. You or your child should be monitored to maintain adequate hydration and consideration should be given to performing laboratory tests on baseline and post-treatment levels (electrolytes, creatinine, and blood urea nitrogen).
If you or your child needs to thicken liquids to swallow them safely, Clensia may neutralize the effect of the thickener.
Taking Clensia with food and drinks
Do not take any solid food from the time you or your child starts taking this medication until after the medical examination.
Pregnancy and breastfeeding
Since the absorption of Clensia in the body is insignificant, Clensia can be used during pregnancy if necessary.
There is no documented experience of the use of this medication during breastfeeding. Since the absorption of this medication in the body of the breastfeeding woman is insignificant, it can be used during breastfeeding if necessary.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Driving and using machines
Clensia does not affect the ability to drive or use machines.
Clensia contains sodium and potassium
This medication contains 3877.8 mg of sodium (main component of table salt/cooking salt) per liter. This is equivalent to 194% of the maximum daily recommended sodium intake for an adult.
This medication contains 11.2 mmol of potassium per liter, which should be taken into account in the treatment of patients with renal insufficiency or with low-potassium diets.
3. How to take Clensia
Use in adults
Follow the administration instructions of this medication exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
This medication is for oral use.
The package contains 4 large sachets A and 4 small sachets B. For a complete treatment cycle, it is necessary to dissolve 8 sachets in 2 liters of water.
Dissolve 2 large sachets A and 2 small sachets B in 1 liter of water.
Before taking Clensia, please read the following instructions carefully. You need to know:
- When to take Clensia
- How to prepare the Clensia solution
- How to drink Clensia
- What to expect to happen
When to take Clensia
Your doctor or nurse should have informed you when to take this medication. Your treatment with this medication should be completed before the clinical examination and can be taken following these instructions:
- Full dose the day before the examination:
Dissolve 4 large sachets A and 4 small sachets B in 2 liters of water and drink them the evening before the examination.
- Split dose:
Dissolve 2 large sachets A and 2 small sachets B in 1 liter of water, drink it the evening before the examination, and dissolve 2 large sachets A and 2 small sachets B in 1 liter of water and drink it the morning of the examination day.
Important: Do not take any solid food from the start of taking Clensia until after the medical examination.
How to prepare Clensia
- Open 2 large sachets A and 2 small sachets B.
- Add the contents of 2 large sachets A and 2 small sachets B to a suitable container.
- Add 1 liter of water to the container and stir until all the powder is dissolved.
- After preparing the solution, it can be stored (keeping it covered) below 25°C until the start of the intestinal preparation. The solution can also be refrigerated in the refrigerator.
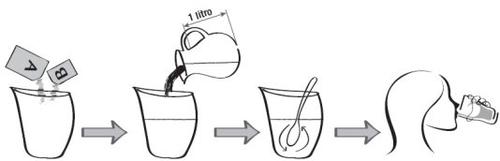
How to take Clensia
- Full dose
The evening before the procedure, dissolve 2 large sachets A and 2 small sachets B in 1 liter of water and drink the Clensia solution over 1 to 1.5 hours. Try to drink 250 ml (two glasses) every 15-20 minutes.
After a 1-2 hour break, dissolve 2 large sachets A and 2 small sachets B in 1 liter of water and drink the solution.
During the course of this treatment, it is recommended to drink an additional 1 liter of clear liquid (eight glasses) to prevent potential fluid loss due to diarrhea and maintain adequate hydration. Water, broth, fruit juices (without pulp), soft drinks, tea, or coffee (without milk) are suitable. These beverages can be taken at any time you wish.
- Split dose
The evening before the diagnostic procedure, dissolve 2 large sachets A and 2 small sachets B in 1 liter of water and drink the solution over 1 to 1.5 hours. Additionally, drink at least 500 ml (four glasses) of clear liquid (water, fruit juice, soft drinks, tea/coffee without milk) throughout the evening.
The morning of the diagnostic procedure, you should prepare the solution following the same process (2 large sachets A and 2 small sachets B dissolved in 1 liter of water), followed by 500 ml (four glasses) of clear liquid (water, fruit juice, soft drinks, tea/coffee without milk).
Leave a gap of at least two hours without drinking before starting the colonoscopy.
What to expect to happen
When you start drinking the Clensia solution, it is essential that you are near a toilet.
At some point, you will start to feel bowel movements. This is very normal and indicates that the solution is working.
The bowel movements will stop soon after you finish drinking it.
If you follow these instructions, your intestine will be clean, and this will help you have a proper examination.
Use in children and adolescents
Adolescents and children from 6 years old
Administer this medication to your child exactly as your doctor has indicated. Consult your doctor if you are unsure.
This medication is for oral use.
Clensia should be administered as a full dose the day before the diagnostic procedure, starting in the late afternoon (4-6 pm).
Dissolve 2 large sachets A and 2 small sachets B in 1 liter of water to obtain the first liter of Clensia solution.
Dissolve 2 large sachets A and 2 small sachets B in another liter of water to prepare the second liter of Clensia solution if necessary.
The dose depends on the child's age and body weight in kilograms.
Children between 6 and less than 12 years old
Weight≤ 25 Kg:give 750 ml of Clensia over 1-2 hours. Additionally, give 375 ml of clear liquid (water, fruit juice, soft drinks, tea without milk) for rehydration.
Weight between25-35 Kg:give 1000 ml of Clensia over 1-2 hours. Additionally, give 500 ml of clear liquid (water, fruit juice, soft drinks, tea without milk) for rehydration.
Weight > 35 Kg:give 1250 ml of Clensia over 1-2 hours. Additionally, give 625 ml of clear liquid (water, fruit juice, soft drinks, tea without milk) for rehydration.
If your child has not had watery and clear stools 3 hours after completing the intestinal solution, give up to 500 ml of additional Clensia.
Adolescents over 12 years old
Weight ≤ 45 Kg:give 1500 ml over 2-3 hours. Additionally, give 750 ml of clear liquid (water, fruit juice, soft drinks, tea without milk) for rehydration.
Weight > 45 Kg:give 1750 ml over 2-3 hours. Additionally, give 875 ml of clear liquid (water, fruit juice, soft drinks, tea without milk) for rehydration.
If the adolescent does not present watery and clear stools 3 hours after completing the intestinal solution, administer additional Clensia up to a maximum cumulative dose of 2000 ml.
If you take more Clensia than you should
If you or your child takes more Clensia than you should, you or your child may experience excessive diarrhea, which can lead to dehydration. Take generous amounts of liquids, especially fruit juices. If you are concerned, contact your doctor or pharmacist.
If you forget to take Clensia
If you or your child forgets to take Clensia, take the dose as soon as you remember.
If several hours have passed since you should have taken it, ask your doctor or pharmacist for advice. It is essential that you complete the administration at least two hours before the examination.
If you interrupt treatment with Clensia
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone experiences them.
It is normal to have diarrhea when you take Clensia.
If you experience any of the following symptoms, stop taking this medication and contact your doctor immediately. You should not take more Clensia until you have consulted your doctor:
- rash or itching
- swelling of the face, ankles, or any other part of the body
- irregular heartbeats
- extreme fatigue
- shortness of breath
Very common side effects (may affect more than 1 in 10 people):
Nausea, abdominal pain, abdominal distension.
Common side effects (may affect more than 1 in 100 people):
Headache, vomiting, anal irritation.
Uncommon side effects (may affect more than 1 in 1000 people):
Temporary increase in blood pressure, stomach pain, taste disturbances, dry mouth, chills, decrease in potassium levels in the blood.
The following side effects have been reported with the use of other polyethylene glycol formulations, but it is not known how often they occur because this cannot be estimated from the available data: allergic reactions (sometimes severe, including anaphylactic shock), dehydration, dizziness, irregular heartbeats, feeling of discomfort, feeling of fainting, sensation of the room spinning (vertigo), flushing, rash, changes in blood electrolyte levels such as decreased or increased sodium, calcium, and chloride, and decreased bicarbonate.
Blood sodium levels may also decrease, particularly in patients taking medications that affect kidney function, such as ACE inhibitors and diuretics used to treat heart disease (see also "Taking Clensia with other medications").
These reactions usually appear on the day of the examination. If they persist, consult your doctor.
Additional side effects in children and adolescents
Very common side effects (may affect more than 1 in 10 people):
Nausea, abdominal pain, abdominal distension, vomiting, fatigue.
Common side effects (may affect more than 1 in 100 people):
Anal irritation, asthenia.
Uncommon side effects (may affect more than 1 in 1000 people):
Headache, chills, diarrhea, abnormal stools, belching.
Reporting side effects
If you or your child experiences any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Clensia
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the box after CAD. The expiration date is the last day of the month indicated.
Store Clensia sachets below 30°C.
After dissolving the contents of the sachets in water, the solution can be stored (keeping it covered) below 25°C. The solution can also be refrigerated in the refrigerator.
Do not store it for more than 24 hours.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the pharmacy's SIGRE point. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Container Content and Additional Information
Clensia Composition
The active ingredients are:
About A
Macrogol (also known as polyethylene glycol) 4000 52,500 g
Anhydrous sodium sulfate 3,750 g
Simethicone 0,080 g
About B
Sodium citrate 1,863 g
Anhydrous citric acid 0,813 g
Sodium chloride 0,730 g
Potassium chloride 0,370 g
The concentration of electrolytes when 2 sachets A and 2 sachets B are dissolved in 1 liter of water is as follows:
Sodium 168.6 mmol/l
Sulfate 52.8 mmol/l
Chloride 34.9 mmol/l
Potassium 11.2 mmol/l
Citrate 21.1 mmol/l
The other excipients are acesulfame potassium (E950), lemon flavor (containing flavoring preparations, natural flavoring substances, sugar glaze with corn starch, gum arabic (E414), maltodextrin).
Product Appearance and Container Content
Clensia is available in a container that contains a single treatment of 8 sachets [4 sachets A (large) and 4 sachets B (small)] and in multiple containers that contain 24, 48, 96, 192 containers with single treatments.
Only some package sizes may be marketed.
Marketing Authorization Holder:
Alfasigma España, S.L.
C/Aribau 195, 4º
08021 Barcelona
Spain
Manufacturer:
Sigmar Italia S.p.A.
Via Sombreno, 11
24011 Almé (BG)
Italy
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany: Clensia Pulver zur Herstellung einer Lösung zum Einnehmen
Czech Republic: Clensia Prášek pro perorální roztok
Slovakia: Clensia Prášok na perorálny roztok
Spain: Clensia Polvo para solución oral
France: Ximepeg Poudre pour solution buvable
Italy: Clensia Polvere per soluzione orale
Netherlands: Clensia Poeder voor drank
Poland: Clensia proszek do sporzadzania roztworu doustnego
Portugal: Clensia Pó para solução oral
Romania: Clensia Pulbere pentru solutie orala
Date of the Last Revision of this Leaflet:April 2024
Other Sources of Information
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Note for Medical Personnel:
Clensia should be administered with caution in fragile patients with poor health or in patients with severe clinical alterations such as:
- swallowing disorders or with a tendency to aspiration or regurgitation
- consciousness disorders
- severe renal insufficiency (creatinine clearance <30 ml min)< li>
- heart failure (NYHA grades III or IV)
- dehydration
- severe acute inflammatory disease.
The presence of dehydration should be corrected before the use of Clensia.
Semi-conscious patients or patients prone to aspiration or regurgitation should be carefully monitored during administration, especially if this takes place via the nasogastric route.
Clensia should not be administered to unconscious patients.
In children unable to drink the necessary amount of solution, a nasogastric tube may be used, with an administration rate of 20-30 ml/minute.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLENSIA POWDER FOR ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 13125 MG + 350.7 MG + 178.5 MG + 46.6 MGActive substance: macrogol, combinationsManufacturer: Lainco S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, gActive substance: macrogol, combinationsManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 13.7 gActive substance: macrogol, combinationsManufacturer: Italfarmaco S.A.Prescription required
Online doctors for CLENSIA POWDER FOR ORAL SOLUTION
Discuss questions about CLENSIA POWDER FOR ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















