CETRAXAL OTICO 3 MG/ML GOTAS OTICAS EN SOLUCION EN ENVASE UNIDOSIS


Cómo usar CETRAXAL OTICO 3 MG/ML GOTAS OTICAS EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Cetraxal ótico 3 mg/ml gotas óticas en solución en envase unidosis
Ciprofloxacino
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted
|
Contenido del prospecto:
- Qué es Cetraxal ótico y para qué se utiliza
- Qué necesita saber antes de empezar a usar Cetraxal ótico
- Cómo usar Cetraxal ótico
- Posibles efectos adversos
- Conservación de Cetraxal ótico
- Contenido del envase e información adicional
1. Qué es Cetraxal ótico y para qué se utiliza
Cetraxal ótico es una solución para uso ótico (en el oído) que contiene ciprofloxacino, que pertenece al grupo de antibióticos denominados fluoroquinolonas. Ciprofloxacino actúa eliminando bacterias que causan infecciones.
Cetraxal ótico está indicado en adultos y niños a partir de 2 años para el tratamiento de otitis externa aguda (infección del oído externo) y otitis media crónica supurada (infección del oído medio) de origen bacteriano.
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas como la gripe o el catarro.
Es importante que siga las instrucciones relativas a la dosis, el intervalo de administración y la duración del tratamiento indicadas por su médico.
No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni a la basura.
2. Qué necesita saber antes de empezar a usar Cetraxal ótico
No use Cetraxal ótico:
Si es alérgico al ciprofloxacino u otras quinolonas o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
- Consulte a su médico o farmacéutico antes de empezar a usar Cetraxal ótico.
- Este medicamento sólo se debe aplicar en el oído. No debe ser ingerido, inyectado o inhalado. No se debe aplicar en el ojo.
- Si una vez iniciado el tratamiento se producen síntomas de urticaria (picor) o erupción cutánea o cualquier otro síntoma alérgico (por ejemplo, hinchazón repentina de la cara, la garganta o los párpados, dificultad respiratoria), interrumpa inmediatamente la medicación y acuda a su médico. Las reacciones graves de hipersensibilidad pueden necesitar tratamiento inmediato de urgencia.
- Aunque no se esperan reacciones de sensibilidad a la luz solar, es recomendable tomar medidas de protección y evitar la exposición excesiva a la luz directa del sol mientras se esté utilizando este medicamento.
- Informe a su médico si los síntomas no mejoran antes de finalizar el tratamiento. Al igual que con otros antibióticos, las infecciones pueden ser a veces causadas por organismos que no son sensibles a ciprofloxacino. En caso de dichas infecciones, el tratamiento apropiado debe ser prescrito por su médico.
Niños:
No hay suficiente experiencia clínica sobre el uso de Cetraxal ótico en niños menores de 2 años, por lo que debe consultar con su médico antes de administrar este medicamento a su hijo si tiene esta edad.
Otros medicamentos y Cetraxal ótico
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No es aconsejable utilizar Cetraxal ótico junto con otros medicamentos por vía ótica.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No es recomendable este medicamento durante el embarazo y la lactancia, por lo que, es su médico quien debe valorar el uso de este medicamento en cada caso.
Conducción y uso de máquinas
No se dispone de datos clínicos al respecto. No obstante, dada la vía de administración, es poco probable que este medicamento influya en la capacidad de conducir vehículos o manejar maquinaria.
3. Cómo usar Cetraxal ótico
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
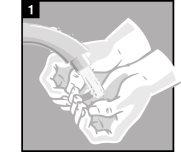
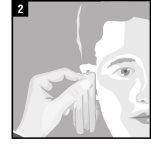
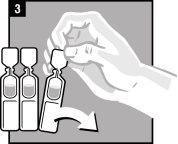
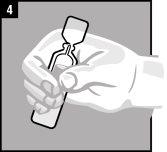
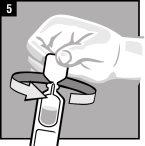
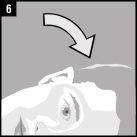
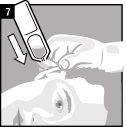
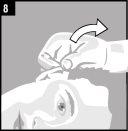
La administración se efectúa directamente en el oído.
Si estima que la acción de este medicamento es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Su médico le indicará la duración del tratamiento con Cetraxal ótico. Para asegurarse de que la infección no reaparece, no interrumpa el tratamiento anticipadamente, incluso si nota mejoría en el oído(s).
La dosis recomendada es:
Adultos
Otitis externa aguda
Administrar el contenido de un envase unidosis (0,4 ml) en el oído afectado dos veces al día, durante 7 días.
Infección crónica supurada del oído medio
Administrar el contenido de un envase unidosis (0,4 ml) en el oído afectado dos veces al día, durante 10 días.
Pacientes de edad avanzada
No es necesario ajustar la dosis en este grupo de pacientes.
Adolescentes y niños mayores de 2 años
No es necesario ajustar la dosis en este grupo de pacientes.
Niños menores de 2 años
No se recomienda su uso, ya que no hay datos disponibles en este grupo de edad.
Pacientes con enfermedades en riñón o hígado
No es necesario ajustar la dosis en este grupo de pacientes.
Forma de administración
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Desechar el envase unidosis tras la administración. | |
|
Si usa más Cetraxal ótico del que debe:
Aunque es difícil que se produzca sobredosis con este medicamento, se recomienda que, en caso de ingestión accidental o sobredosis, consulte inmediatamente al médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Cetraxal ótico
No administre una dosis doble para compensar las dosis olvidadas.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Las reacciones adversas descritas son pocofrecuentes (pueden afectar hasta 1 de cada 100 personas)
-Mareo, dolor de cabeza, vértigo.
-Dolor de oído, paso del producto del oído a la boca.
-Eczema, erupción cutánea o picor, sensación de pinchazo en el momento de la aplicación.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Cetraxal ótico
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de EXP. La fecha de caducidad es el último día del mes que se indica.
Después de la apertura del envase unidosis: usar inmediatamente y desechar el envase unidosis después de la administración.
Este medicamento no requiere condiciones especiales de conservación. Conservar los envases unidosis dentro del sobre protector, preferiblemente dentro de la caja.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Cetraxal ótico
- El principio activo es ciprofloxacino. Cada mililitro de solución contiene 3 mg de ciprofloxacino.
- Los demás componentes son: ácido láctico 90%, povidona, glucosa anhidra, solución de hidróxido sódico y agua purificada.
Aspecto del producto y contenido del envase
Cetraxal ótico es un medicamento que se presenta en forma de gotas en solución para la administración en el oído. Cada envase unidosis contiene 0,4 ml de solución estéril y transparente.
Titular de la autorización de comercialización y responsable de la fabricación
Laboratorios Salvat, S.A.
C/ Gall 30 – 36 - 08950
Esplugues de Llobregat
Barcelona - España
Fecha de la última revisión de este prospecto: julio 2006.
- País de registro
- Precio medio en farmacia6.01 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CETRAXAL OTICO 3 MG/ML GOTAS OTICAS EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: LÍQUIDO OTICO, 3 mg/mlPrincipio activo: CiprofloxacinoFabricante: Zambon S.A.U.Requiere recetaForma farmacéutica: LÍQUIDO OTICO, 3 mgPrincipio activo: CiprofloxacinoFabricante: Laboratorios Salvat S.A.Requiere recetaForma farmacéutica: LÍQUIDO OTICO, 1 mg/0,5 mlPrincipio activo: CiprofloxacinoFabricante: Neuraxpharm Spain S.L.Requiere receta
Médicos online para CETRAXAL OTICO 3 MG/ML GOTAS OTICAS EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CETRAXAL OTICO 3 MG/ML GOTAS OTICAS EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes