
CASPOFUNGINA LORIEN 50 mg POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION

How to use CASPOFUNGINA LORIEN 50 mg POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Caspofungin Lorien powder for concentrate for solution for infusion is and what it is used for
- What you need to know before you use Caspofungin Lorien powder for concentrate for solution for infusion
- How to use Caspofungin Lorien powder for concentrate for solution for infusion
- Possible side effects
- Conservation of Caspofungin Lorien powder for concentrate for solution for infusion
- Container Content and Additional Information
Introduction
Package Leaflet: Information for the User
Caspofungin Lorien 50 mg powder for concentrate for solution for infusion EFG
Caspofungin
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What Caspofungin Lorien powder for concentrate for solution for infusion is and what it is used for
- What you need to know before you use Caspofungin Lorien powder for concentrate for solution for infusion
- How to use Caspofungin Lorien powder for concentrate for solution for infusion
- Possible side effects
5 Conservation of Caspofungin Lorien powder for concentrate for solution for infusion
- Contents of the pack and further information
1. What Caspofungin Lorien powder for concentrate for solution for infusion is and what it is used for
What Caspofungin powder for concentrate for solution for infusion is
Caspofungin powder for concentrate for solution for infusion contains a medicine called caspofungin. This belongs to a group of medicines called antifungals.
What Caspofungin powder for concentrate for solution for infusion is used for
Caspofungin is used to treat the following infections in children, adolescents, and adults:
- severe fungal infections in your tissues or organs (called “invasive candidiasis”). This infection is caused by fungal cells (yeast) called Candida. People who may get this type of infection include those who have just had an operation or those whose immune system is weakened. Fever and chills that do not respond to antibiotic treatment are the most common symptoms of this type of infection.
infections in your nose, sinuses, or lungs (called “invasive aspergillosis”) if other antifungal treatments have not worked or have caused side effects. This infection is caused by molds called Aspergillus.
- Possible fungal infections if you have a fever and a low white blood cell count that has not improved with antibiotic treatment. People who are at risk of getting a fungal infection include those who have just had an operation or those whose immune system is weakened.
How Caspofungin powder for concentrate for solution for infusion works
Caspofungin makes fungal cells fragile and prevents the fungus from growing properly. This prevents the infection from spreading and gives the body's natural defenses a chance to get rid of the infection completely.
2. What you need to know before you use Caspofungin Lorien powder for concentrate for solution for infusion
Do not use Caspofungin Lorien powder for concentrate for solution for infusion
- if you are allergic to caspofungin or any of the other ingredients of this medicine (listed in section 6).
If you are not sure, talk to your doctor, pharmacist, or nurse before you start using your medicine.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Caspofungin Lorien powder for concentrate for solution for infusion if:
- you are allergic to any other medicine.
- you have ever had liver problems; you may need a different dose of this medicine.
- you are already taking cyclosporin (which is used to prevent organ transplant rejection or to suppress your immune system), as your doctor will probably need to do extra blood tests during your treatment.
- you have ever had any other medical problem.
Caspofungin Lorien may also cause severe skin reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).
If any of the above applies to you (or you are not sure), talk to your doctor, pharmacist, or nurse before you start using Caspofungin Lorien.
Using Caspofungin Lorien powder for concentrate for solution for infusion with other medicines
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines. This includes medicines that you buy without a prescription, including herbal remedies.
This is because Caspofungin Lorien may affect the way other medicines work. Other medicines may also affect the way Caspofungin Lorien works.
Tell your doctor, pharmacist, or nurse if you are taking any of the following medicines:
- cyclosporin or tacrolimus (which are used to prevent organ transplant rejection or to suppress your immune system), as your doctor will probably need to do extra blood tests during your treatment.
- certain anti-HIV medicines such as efavirenz or nevirapine.
- phenytoin or carbamazepine (which are used to treat seizures).
- dexamethasone (a steroid).
- rifampicin (an antibiotic).
If any of the above applies to you (or you are not sure), talk to your doctor, pharmacist, or nurse before you start using Caspofungin Lorien.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
- Caspofungin Lorien has not been studied in pregnant women. It should only be used during pregnancy if the potential benefit justifies the potential risk to the unborn baby.
- Women who use Caspofungin Lorien must not breastfeed.
Driving and using machines
There is no information to suggest that Caspofungin Lorien affects your ability to drive or use machines.
3. How to use Caspofungin Lorien powder for concentrate for solution for infusion
Caspofungin will always be prepared and given to you by a healthcare professional. You will be given Caspofungin:
- once a day.
- by slow injection into a vein (intravenous infusion).
- over about 1 hour.
Your doctor will decide how long you should be treated with Caspofungin and how much Caspofungin you should be given each day. Your doctor will monitor whether the effect of the medicine is adequate. If you weigh more than 80 kg, you may need a different dose.
Use in children and adolescents
The dose for children and adolescents may be different from the dose in adults.
If you use more Caspofungin powder for concentrate for solution for infusion than you should
Your doctor will decide how much Caspofungin you need and for how long each day. If you are worried that you may have been given too much Caspofungin, tell your doctor or nurse straight away.
In case of overdose or accidental ingestion, contact your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or nurse straight away if you notice any of the following side effects – you may need urgent medical treatment:
- rash, itching, feeling of warmth, swelling of your face, lips or throat or difficulty breathing: you may be having a histamine reaction to the medicine.
- difficulty breathing with wheezing or worsening of a rash that you already have: you may be having an allergic reaction to the medicine.
- cough, severe breathing difficulties: if you are an adult and have invasive aspergillosis, you may experience a severe breathing problem that could lead to respiratory failure.
- rash, skin peeling, mucosal ulcers, redness, large areas of skin peeling
As with any prescription medicine, some side effects may be serious. Ask your doctor for more information.
Other side effects in adults include:
Common: may affect up to 1 in 10 people:
- decrease in hemoglobin (decrease in the substance that carries oxygen in the blood), decrease in white blood cells.
- decrease in albumin (a type of protein) in your blood, decrease in potassium or low potassium levels in the blood.
- headache.
- vein inflammation.
- shortness of breath.
- diarrhea, nausea, or vomiting.
- changes in some laboratory blood tests (such as increased values of some liver tests).
- itching, rash, redness of the skin or more sweating than usual.
- joint pain.
- chills, fever.
- itching at the injection site.
Uncommon: may affect up to 1 in 100 people:
- changes in some laboratory blood tests (including blood coagulation disorders, platelets, red blood cells, and white blood cells).
- loss of appetite, increase in body fluid, imbalance of body salts, high blood sugar, low calcium levels in the blood, low magnesium levels in the blood, increase in the level of acids in the blood.
- disorientation, feeling nervous, inability to sleep.
- feeling dizzy, decreased sensations or sensitivity (especially in the skin), agitation, feeling sleepy, change in the way things taste, tingling or numbness.
- blurred vision, increased tears, swollen eyelid, yellow coloration of the white of the eyes.
- feeling of rapid or irregular heartbeats, rapid heartbeat, irregular heartbeat, abnormal heart rhythm, heart failure.
- flushing, hot flashes, high blood pressure, low blood pressure, redness along a vein that is very sensitive to touch.
- tightness in the muscle bands around the airways leading to wheezing or cough, rapid breathing rate, shortness of breath that wakes you up, lack of oxygen in the blood, abnormal breathing sounds, crackling sounds in the lungs, wheezing, nasal congestion, cough, sore throat.
- abdominal pain, pain in the upper abdomen, swelling, constipation, difficulty swallowing, dry mouth, indigestion, passing gas, stomach upset, swelling due to fluid accumulation around the abdomen.
- decrease in bile flow, increase in liver size, yellow coloration of the skin and/or the white of the eyes, liver injury caused by a drug or chemical, liver disorder.
- abnormal skin tissue, generalized itching, hives, rash of varying appearance, abnormal skin, red spots, often with itching, on arms and legs and sometimes on the face and the rest of the body.
- back pain, pain in an arm or leg, bone pain, muscle pain, muscle weakness.
- loss of kidney function, sudden loss of kidney function.
- pain at the catheter site, symptoms at the injection site (redness, hard lump, pain, swelling, irritation, rash, hives, fluid leakage from the catheter to the tissue), vein inflammation at the injection site.
- increase in blood pressure and changes in some laboratory blood tests (such as kidney electrolyte tests and coagulation tests), increase in levels of medicines that you are taking that suppress your immune system.
- chest discomfort, chest pain, feeling of change in body temperature, feeling unwell, general pain, swelling of the face, swelling of the ankles, hands, or feet, swelling, pain when touched
Other side effects in children and adolescents
Very common: may affect more than 1 in 10 people:
- fever.
Common: may affect up to 1 in 10 people:
- headache.
- rapid heartbeat.
- flushing, low blood pressure.
- changes in some laboratory blood tests (increased values of some liver tests).
- itching, rash.
- pain at the catheter site.
- chills.
- changes in some laboratory blood tests.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency's Pharmacovigilance System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Conservation of Caspofungin Lorien powder for concentrate for solution for infusion
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and vial after EXP. The expiry date is the last day of the month shown.
Store and transport in a refrigerator (between 2 °C and 8 °C).
Once Caspofungin Lorien has been prepared, it should be used immediately. This is because it does not contain any preservative to prevent bacterial growth. Only a trained healthcare professional who has read the complete instructions should prepare the medicine (see “Instructions for reconstituting and diluting Caspofungin Lorien” below).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of Caspofungina Lorien
- The active ingredient is caspofungina. Each vial contains 50 mg of caspofungina.
After reconstitution, each ml of concentrate contains 5.2 mg of caspofungina
- The other components are sucrose, mannitol (E421), succinic acid (E363), and sodium hydroxide (for pH adjustment) (E524).
Product Appearance and Container Content
Caspofungina Lorien is a compact powder, white to off-white in color.
Each container contains one vial of powder.
Marketing Authorization Holder
Laboratorios Lorien S.L
Av. Josep Tarradellas, 8. Ático 1ª
08029 Barcelona
Spain
Manufacturer
Laboratori Fundació DAU
C/ C, 12-14 Pol. Ind. Zona Franca
08040 Barcelona
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Member State | Medicinal Product Name |
Netherlands | Caspofungine Regiomedica 50 mg & 70 mg, powder for concentrate for solution for infusion |
Germany | Caspofungin Regiomedica 50 mg & 70 mg powder for concentrate for solution for infusion |
Denmark | Caspofungin Regiomedica 50 mg & 70 mg powder for concentrate for infusion solution |
Spain | Caspofungina Lorien 50 mg & 70 mg, powder for concentrate for solution for perfusion EFG |
Finland | Kaspofungiinia Regiomedica 50 mg & 70 mg kuiva-aine välikonsentraatiksi infuusionestettä varten, liuos |
France | Caspofungine Regiomedica 50 mg & 70 mg powder for solution to be diluted for perfusion |
Italy | Caspofungin Regiomedica |
Norway | Caspofungin Regiomedica |
Sweden | Kaspofungin Regiomedica |
United Kingdom | Caspofungin 50 mg & 70 mg powder for concentrate for solution for infusion |
Date of the last revision of this leaflet: September 2016
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
------------------------------------------------------------------------------------------------------------------
This information is intended exclusively for healthcare professionals:
Instructions for the reconstitution and dilution of Caspofungina Lorien 50 mg:
Reconstitution of Caspofungina Lorien 50 mg
DO NOT USE DILUENTS CONTAINING GLUCOSE, as Caspofungina Lorien is not stable in diluents containing glucose. DO NOT MIX OR CO-ADMINISTER Caspofungina Lorien WITH ANY OTHER MEDICINAL PRODUCT, as there are no data available on the compatibility of Caspofungina Lorien with other substances, additives, or intravenous medicinal products. The infusion solution should be inspected visually for the presence of particulate matter or a change in color.
INSTRUCTIONS FOR USE IN ADULT PATIENTS
Step 1 Reconstitution of the vials
To reconstitute the powder, bring the vial to room temperature and add aseptically 10.5 ml of water for injections. The concentration of the reconstituted vials will be 5.2 mg/ml.
The compact white to off-white lyophilized powder will dissolve completely. Mix gently until a clear, colorless, or slightly yellowish solution is obtained. The reconstituted solutions should be inspected visually for the presence of particulate matter or a change in color. The reconstituted solution can be stored for a maximum of 24 hours at a temperature of 25°C or below.
Step 2 Addition of Caspofungina Lorien reconstituted to the patient's infusion solution
The diluents for the final infusion solution are: sodium chloride injection solution or Ringer's lactate solution. The infusion solution is prepared by adding aseptically the appropriate amount of reconstituted concentrate (as shown in the following table) to a 250 ml infusion bag or bottle. For daily doses of 50 mg or 35 mg, reduced-volume infusions in 100 ml may be used if medically necessary. Do not use if the solution is turbid or has precipitated.
PREPARATION OF THE INFUSION SOLUTION IN ADULTS
DOSE* | Volume of Caspofungina Lorien reconstituted to be transferred to an intravenous bag or bottle | Standard preparation(Caspofungina Lorien reconstituted added to 250 ml) final concentration | Reduced-volume infusion (Caspofungina Lorien reconstituted added to 100 ml) final concentration |
50 mg | 10 ml | 0.20 mg/ml | - |
50 mg in reduced volume | 10 ml | - | 0.47 mg/ml |
35 mg for moderate hepatic impairment (from a 50 mg vial) | 7 ml | 0.14 mg/ml | - |
35 mg for moderate hepatic impairment (from a 50 mg vial) in reduced volume | 7 ml | - | 0.34 mg/ml |
*10.5 ml should be used for the reconstitution of all vials
INSTRUCTIONS FOR USE IN PEDIATRIC PATIENTS
Calculation of body surface area (BSA) for pediatric dosing
Before preparing the infusion, calculate the patient's body surface area (BSA) using the following formula: (Mosteller formula1)
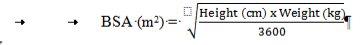
Preparation of the 70 mg/m2infusion for pediatric patients >3 months (using a 50 mg vial)
- Determine the actual loading dose to be used in the pediatric patient using the patient's BSA (as calculated above) and the following equation: BSA (m2) x 70 mg/m2 = loading dose. The maximum loading dose on day 1 should not exceed 70 mg, regardless of the calculated dose for the patient.
- Allow the Caspofungina Lorien vial to reach room temperature.
- Add aseptically 10.5 ml of water for injections. aThis reconstituted solution can be stored for up to 24 hours at a temperature of 25°C or below. bThis will result in a final concentration of caspofungina in the vial of 5.2 mg/ml.
- Withdraw from the vial a volume of the medicinal product equal to the calculated loading dose (Step 1). Transfer aseptically this volume (ml) of Caspofungina Lorien reconstituted to an intravenous bag or bottle containing 250 ml of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution. Alternatively, the volume (ml) of Caspofungina Lorien reconstituted can be added to a reduced volume of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution, without exceeding a final concentration of 0.5 mg/ml. This infusion solution should be used within 24 hours if stored at a temperature of 25°C or below or within 48 hours if stored refrigerated between 2 and 8°C.
Preparation of the 50 mg/m2infusion for pediatric patients >3 months (using a 50 mg vial)
- Determine the actual daily maintenance dose to be used in the pediatric patient using the patient's BSA (as calculated above) and the following equation: BSA (m2) x 50 mg/m2 = daily maintenance dose. The daily maintenance dose should not exceed 70 mg, regardless of the calculated dose for the patient.
- Allow the Caspofungina Lorien vial to reach room temperature.
- Add aseptically 10.5 ml of water for injections. aThis reconstituted solution can be stored for up to 24 hours at a temperature of 25°C or below. bThis will result in a final concentration of caspofungina in the vial of 5.2 mg/ml.
- Withdraw from the vial a volume of the medicinal product equal to the calculated daily maintenance dose (Step 1). Transfer aseptically this volume (ml) of Caspofungina Lorien reconstituted to an intravenous bag or bottle containing 250 ml of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution. Alternatively, the volume (ml) of Caspofungina Lorien reconstituted can be added to a reduced volume of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution, without exceeding a final concentration of 0.5 mg/ml. This infusion solution should be used within 24 hours if stored at a temperature of 25°C or below or within 48 hours if stored refrigerated between 2 and 8°C.
Preparation Notes:
- The white to off-white compact powder will dissolve completely. Mix gently until a clear, colorless, or slightly yellowish solution is obtained.
- Inspect the reconstituted solution visually for the presence of particulate matter or a change in color during reconstitution and before infusion. Do not use if the solution is turbid or has precipitated.
- Caspofungina Lorien is formulated to provide the full dose of the vial as stated in the package insert (50 mg) when 10 ml is withdrawn from the vial.
1 Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med1987 Oct 22;317(17): 1098 (letter)
Instructions for the reconstitution and dilution of Caspofungina Lorien 70 mg:
Reconstitution of Caspofungina Lorien 70 mg
DO NOT USE DILUENTS CONTAINING GLUCOSE, as Caspofungina Lorien is not stable in diluents containing glucose. DO NOT MIX OR CO-ADMINISTER Caspofungina Lorien WITH ANY OTHER MEDICINAL PRODUCT, as there are no data available on the compatibility of Caspofungina Lorien with other substances, additives, or intravenous medicinal products. The infusion solution should be inspected visually for the presence of particulate matter or a change in color.
INSTRUCTIONS FOR USE IN ADULT PATIENTS
Step 1 Reconstitution of the vials
To reconstitute the powder, bring the vial to room temperature and add aseptically 10.5 ml of water for injections. The concentration of the reconstituted vials will be 7.2 mg/ml.
The compact white to off-white lyophilized powder will dissolve completely. Mix gently until a clear, colorless, or slightly yellowish solution is obtained. The reconstituted solutions should be inspected visually for the presence of particulate matter or a change in color. The reconstituted solution can be stored for a maximum of 24 hours at a temperature of 25°C or below.
Step 2 Addition of Caspofungina Lorien reconstituted to the patient's infusion solution
The diluents for the final infusion solution are: sodium chloride injection solution or Ringer's lactate solution. The infusion solution is prepared by adding aseptically the appropriate amount of reconstituted concentrate (as shown in the following table) to a 250 ml infusion bag or bottle. For daily doses of 50 mg or 35 mg, reduced-volume infusions in 100 ml may be used if medically necessary. Do not use if the solution is turbid or has precipitated.
PREPARATION OF THE INFUSION SOLUTION IN ADULTS
DOSE* | Volume of Caspofungina Lorien reconstituted to be transferred to an intravenous bag or bottle | Standard preparation(Caspofungina Lorien reconstituted added to 250 ml) final concentration | Reduced-volume infusion (Caspofungina Lorien reconstituted added to 100 ml) final concentration |
70 mg | 10 ml | 0.28 mg/ml | Not recommended |
70 mg (from two 50 mg vials)** | 14 ml | 0.28 mg/ml | Not recommended |
35 mg for moderate hepatic impairment (from a 70 mg vial) | 5 ml | 0.14 mg/ml | 0.34 mg/ml |
*10.5 ml should be used for the reconstitution of all vials
**If the 70 mg vial is not available, the 70 mg dose can be prepared from two 50 mg vials
INSTRUCTIONS FOR USE IN PEDIATRIC PATIENTS
Calculation of body surface area (BSA) for pediatric dosing
Before preparing the infusion, calculate the patient's body surface area (BSA) using the following formula: (Mosteller formula2)
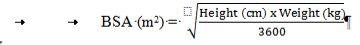
Preparation of the 70 mg/m2infusion for pediatric patients >3 months (using a 70 mg vial)
- Determine the actual loading dose to be used in the pediatric patient using the patient's BSA (as calculated above) and the following equation: BSA (m2) x 70 mg/m2 = loading dose. The maximum loading dose on day 1 should not exceed 70 mg, regardless of the calculated dose for the patient.
- Allow the Caspofungina Lorien vial to reach room temperature.
- Add aseptically 10.5 ml of water for injections. aThis reconstituted solution can be stored for up to 24 hours at a temperature of 25°C or below. bThis will result in a final concentration of caspofungina in the vial of 7.2 mg/ml.
- Withdraw from the vial a volume of the medicinal product equal to the calculated loading dose (Step 1). Transfer aseptically this volume (ml) of Caspofungina Lorien reconstituted to an intravenous bag or bottle containing 250 ml of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution. Alternatively, the volume (ml) of Caspofungina Lorien reconstituted can be added to a reduced volume of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution, without exceeding a final concentration of 0.5 mg/ml. This infusion solution should be used within 24 hours if stored at a temperature of 25°C or below or within 48 hours if stored refrigerated between 2 and 8°C.
Preparation of the 50 mg/m2infusion for pediatric patients >3 months (using a 70 mg vial)
- Determine the actual daily maintenance dose to be used in the pediatric patient using the patient's BSA (as calculated above) and the following equation: BSA (m2) x 50 mg/m2 = daily maintenance dose.
The daily maintenance dose should not exceed 70 mg, regardless of the calculated dose for the patient.
- Allow the Caspofungina Lorien vial to reach room temperature.
- Add aseptically 10.5 ml of water for injections. aThis reconstituted solution can be stored for up to 24 hours at a temperature of 25°C or below. bThis will result in a final concentration of caspofungina in the vial of 7.2 mg/ml.
- Withdraw from the vial a volume of the medicinal product equal to the calculated daily maintenance dose (Step 1). Transfer aseptically this volume (ml) of Caspofungina Lorien reconstituted to an intravenous bag or bottle containing 250 ml of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution. Alternatively, the volume (ml) of Caspofungina Lorien reconstituted can be added to a reduced volume of sodium chloride injection solution 0.9%, 0.45%, or 0.225%, or Ringer's lactate solution, without exceeding a final concentration of 0.5 mg/ml. This infusion solution should be used within 24 hours if stored at a temperature of 25°C or below or within 48 hours if stored refrigerated between 2 and 8°C.
Preparation Notes:
- The white to off-white compact powder will dissolve completely. Mix gently until a clear, colorless, or slightly yellowish solution is obtained.
- Inspect the reconstituted solution visually for the presence of particulate matter or a change in color during reconstitution and before infusion. Do not use if the solution is turbid or has precipitated.
- Caspofungina Lorien is formulated to provide the full dose of the vial as stated in the package insert (70 mg) when 10 ml is withdrawn from the vial.
2 Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med1987 Oct 22;317(17): 1098 (letter)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CASPOFUNGINA LORIEN 50 mg POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSIONDosage form: INJECTABLE INFUSION, UnknownActive substance: caspofunginManufacturer: Merck Sharp & Dohme B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, UnknownActive substance: caspofunginManufacturer: Merck Sharp & Dohme B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 50 mgActive substance: caspofunginManufacturer: Demo S.A. Pharmaceutical IndustryPrescription required
Online doctors for CASPOFUNGINA LORIEN 50 mg POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION
Discuss questions about CASPOFUNGINA LORIEN 50 mg POWDER FOR CONCENTRATE FOR SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















