
BICNU 100 MG POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION EFG

Cómo usar BICNU 100 MG POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
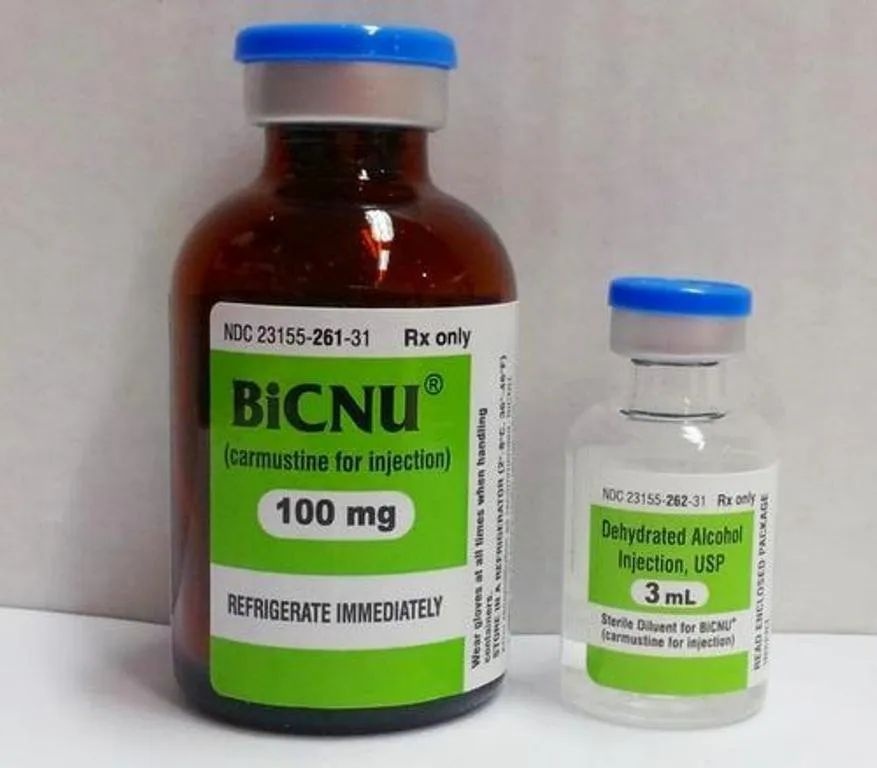
BiCNU 100 mg Polvo y disolvente para solución para perfusión EFG
Carmustina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Bicnu y para qué se utiliza
- Qué necesita saber antes de empezar usar Bicnu
- Cómo usar Bicnu
- Posibles efectos adversos
- Conservación de Bicnu
- Contenido del envase e información adicional
1. Qué es Bicnu y para qué se utiliza
Bicnu es un medicamento que contiene carmustina. La carmustina pertence al grupo de substancias anticancerígenas conocidas como nitrosoureas, que actúan ralentizando el crecimiento de las células cancerígenas.
Bicnu se usa como terapia paliativa (aliviando y previniendo el sufrimiento de los pacientes) como agente único o en terapia de combinación establecida con otras substancias anticancerígenas aprobadas en ciertos tipos de cáncer, como:
- Tumores cerebrales - glioblastoma, meduloblastoma, astrocitoma y tumores cerebrales metastáticos
- Mieloma múltiple (tumor maligno en desarrollo desde la médula ósea)
- Enfermedad de Hodgkin (tumor linfoide)
- Linfoma no Hodgkin (tumor linfoide)
La carmustina también se utiliza como tratamiento de acondicionamiento previo al trasplante autólogo de células madre (un procedimiento en el que una persona recibe células madre hematopoyéticas, que producen cualquier tipo de célula sanguínea) en trastornos hematológicos malignos del sistema linfático como el linfoma de Hodgkin y el linfoma no Hodgkin
2. Qué necesita saber antes de empezar a tomar Bicnu
No use Bicnu
- si es alérgico a la carmustina, a otras nitrosoureas o a cualquier otro ingrediente de este medicamento (listados en la sección 6);
-Bicnu no debe administrarse a pacientes que tengan un número reducido de plaquetas (trombocitos), de glóbulos blancos (leucocitos) o de glóbulos rojos (eritrocitos), tanto si es a consecuencia de la quimioterapia como si es por otras causas;
-si padece insuficiencia renal grave;
-en niños y adolescentes;
-si está embarazada o en periodo de lactancia.
Advertencias y precauciones
Consulte con su médico, farmacéutico o enfermero antes de empezar a usar Bicnu.
Puesto que la mayor toxicidad de este medicamento es la supresión tardía de médula ósea, su médico controlará su recuento sanguíneo al menos durante las 6 semanas siguientes a la administración de la dosis. A la dosis recomendada, los ciclos de Bicnu no se administrarán con una frecuencia mayor a 6 semanas. La dosis se confirmará con el recuento sanguíneo.
Antes del tratamiento, se comprobará tanto su función hepática como la renal y ambas serán vigiladas regularmente durante el tratamiento.
Debido a que el uso de Bicnu puede conllevar daños pulmonares, se efectuarán radiografías de la región pectoral y pruebas de la función pulmonar (por favor, vea la sección “Posibles efectos adversos”)
El tratamiento con altas dosis de carmustina (hasta 600 mg/m2) solo se realiza en combinación con el posterior trasplante de células madre. Estas dosis altas pueden incrementar la frecuencia o la gravedad de toxicidades pulmonares, renales, hepáticas, cardiacas y gastrointestinales así como de infecciones y alteraciones en el equilibrio electrolítico (bajos niveles en sangre de potasio, magnesio y fosfato).
El dolor abdominal (enterocolitis neutropénica) puede ocurrir como un acontecimiento adverso relacionado con la terapia con agentes quimioterapéuticos.
Los pacientes que padecen múltiples afecciones simultáneamente y tienen un estado de enfermedad más precario tienen un mayor riesgo de sufrir efectos adversos. Esto es especialmente importante para los pacientes de edad avanzada.
Su médico le aconsejará sobre la posibilidad de daño pulmonar que puede aparecer debido al uso Bicnu y sobre las reacciones alérgicas y sus síntomas. Si dichos síntomas aparecieran, debe consultar a su médico (vea sección 4)
Uso de Bicnu con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar otros medicamentos, incluidos medicamentos sin receta médica, como:
- Fenitoína, utilizado para la epilepsia
- Cimetidina, utilizado para problemas estomacales como la indigestión
- Digoxina, utilizado si tiene un ritmo cardíaco anormal
- Melfalán, fármaco anticancerígeno
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo y fertilidad
Bicnu no debería ser utilizado durante el embarazo ya que puede dañar al feto. Por lo tanto, Bicnu no debe administrarse normalmente en mujeres embarazadas. Si se administrara durante el embarazo, la paciente debe estar informada sobre el riesgo potencial para el feto. Se aconseja a las mujeres en edad fértil que eviten quedarse embarazadas.
Los pacientes masculinos deben utilizar medidas anticonceptivas adecuadas durante el tratamiento con Bicnu durante al menos 6 meses.
Lactancia
No debe amamantar mientras esté tomando este medicamento.
Conducción y uso de máquinas
Se desconoce el efecto de este medicamento sobre la conducción y el uso de maquinaria. Debe consultar con su médico antes de conducir o utilizar cualquier herramienta o maquinaria porque la cantidad de alcohol de este medicamento puede disminuir su capacidad para conducir o utilizar maquinaria.
Bicnu contiene propilenglicol
El propilenglicol de este medicamento puede tener los mismos efectos que el consumo de alcohol y aumentar la probabilidad de efectos secundarios.
No use este medicamento en niños menores de 5 años.
Use este medicamento solo si se lo recomienda un médico. Su médico puede realizar controles adicionales mientras esté tomando este medicamento
3. Cómo usar Bicnu
Bicnu será siempre administrado por profesionales sanitarios con experiencia en el uso de agentes anticancerígenos.
Este medicamento se administra por vía intravenosa.
Adultos
La dosis depende de su condición médica, del tamaño de su cuerpo y de la respuesta al tratamiento. Normalmente se administra al menos cada 6 semanas. La dosis recomendada de Bicnu como agente único en pacientes que no han sido tratados anteriormente es de 150 a 200 mg/m2 por vía intravenosa cada 6 semanas. Esto se puede administrar como una única dosis o dividirse en dos inyecciones diarias de 75 a 100 mg/m2 en dos días consecutivos. La dosis también dependerá de si Bicnu es administrado con otros fármacos anticancerígenos.
Las dosis se ajustarán dependiendo de cómo responda usted al tratamiento.
La dosis recomendada de carmustina suministrada en combinación con otros agentes quimioterapéuticos antes de un trasplante de células progenitoras hematopoyéticas es de 300- 600 mg/m2 por vía intravenosa.
Su recuento sanguíneo será controlado con frecuencia para evitar la toxicidad en la médula ósea y ajustar la dosis si fuera necesario.
Método de administración
La administración intravenosa debe llevarse a cabo mediante perfusión.
Bicnu se administra en la vena mediante un goteo en un periodo de una a dos horas. El tiempo de perfusión no debe ser menos de una hora para evitar quemaduras y dolor en la zona de la inyección. La zona de la inyección se controlará durante la administración.
La duración del tratamiento la determinará el médico y puede variar para cada paciente.
Uso en pacientes de edad avanzada
Bicnu se puede utilizar con precaución en pacientes de edad avanzada. La función renal se controlará cuidadosamente.
En los pacientes de edad avanzada, la incidencia de la inflamación de las membranas mucosas de la boca (mucositis oral) es mayor cuando se administra una dosis alta de carmustina
Si usa más Bicnu del que debe
Puesto que será el médico o enfermero quien le administre este medicamento, es poco probable que reciba una dosis equivocada. Informe a su médico o enfermero si tiene alguna duda sobre la cantidad de medicamento que está recibiendo.
Si tiene cualquier otra duda sobre el uso de este medicamento, consulte a su médico, farmacéutico o enfermero.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Intoxicación Toxicológica. Teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico o enfermero inmediatamente si experimenta alguno de los siguientes síntomas:
Cualquier jadeo repentino, dificultad para respirar, hinchazón de los párpados, cara o labios, erupción en la piel o picor (sobre todo si afecta a todo el cuerpo), y la sensación de que se va a desmayar. Estos pueden ser signos de una reacción alérgica grave.
Bicnu puede causar los siguientes efectos adversos:
Muy frecuentes(pueden afectar a más de 1 de cada 10 persona)
- Mielosupresión tardía (disminución de las células sanguíneas en la médula ósea);
- Ataxia (falta de coordinación voluntaria de los movimientos musculares);
- Mareos;
- Dolor de cabeza
- Enrojecimiento transitorio del ojo, visión borrosa, sangrado de retina;
- Hipotensión (descenso de la presión sanguínea) en terapias con dosis elevadas;
- Flebitis (inflamación de las venas);
- Trastornos respiratorios (trastornos pulmonares) con problemas de respiración;
- Náuseas y vómitos severos; empezando a las 2-4 horas desde la administración y con una duración de hasta 4-6 horas;
- Cuando se usa en la piel, inflamación cutánea (dermatitis)
- Un contacto accidental con la piel puede causar hiperpigmentación transitoria (oscurecimiento de una zona de la piel o de las uñas)
Frecuentes(pueden afectar a 1 de cada 10 personas)
- Leucemia aguda y displasias de médula ósea (desarrollo anormal de la médula ósea) tras un uso a largo plazo; Pueden aparecer los siguientes síntomas: sangrado de encías, dolor de huesos, fiebre, infecciones frecuentes, hemorragias nasales frecuentes o graves, bultos debidos a la inflamación de los ganglios linfáticos en y alrededor del cuello, antebrazo, abdomen o ingle, piel pálida, dificultad para respirar, debilidad, fatiga o falta general de energía;
- Anemia (disminución de la cantidad de glóbulos rojos en la sangre);
- Encefalopatía (trastorno del cerebro) en terapias con dosis elevadas;
- Pérdida de apetito (anorexia);
- Estreñimiento;
- Diarrea;
- Inflamación de la boca y de los labios;
- Toxicidad hepática reversible en terapias con dosis elevadas, hasta 60 días después de la administración. Esto puede manifestarse por un aumento de las enzimas hepáticas y de la bilirrubina.
- Alopecia (pérdida de cabello);
- Enrojecimiento de la piel;
- Reacciones en la zona de la inyección
Raros(pueden afectar hasta a 1 de cada 1.000 personas)
- Enfermedad veno-oclusiva (bloqueo progresivo de las venas) en terapias con dosis elevadas; en la que se bloquean venas muy pequeñas del hígado. Son posibles los siguientes síntomas: acumulación de líquido en el abdomen, agrandamiento del bazo, sangrado severo del esófago, coloración amarillenta de la piel y piel blanca de los ojos;
- Problemas respiratorios debido a un tipo de enfermedad pulmonar en la que el tejido tiene cicatrices por fibrosis intersticial (con dosis más bajas);
- Toxicidad renal;
- Ginecomastia (crecimiento de los pechos en los hombres);
- Inflamación del nervio óptico y la retina adyacente en el ojo;
- Sangrado del tracto intestinal
Muy raros(pueden afectar hasta a 1 de cada 10.000 personas)
- Inflamación de la pared venosa con trombosis asociada (tromboflebitis)
Frecuencia no conocida(la frecuencia no puede estimarse a partir de los datos disponibles)
- Reacciones alérgicas
- Dolor muscular;
- Tumores secundarios (cánceres causados por radiación o por quimioterapia);
- Convulsiones (ataques) incluyendo “status epilepticus”;
- Daños en el tejido debido a una fuga en la zona inyectada;
- Infertilidad;
- Insuficiencia en el desarrollo embrionario / fetal en mujeres embarazadas;
- Cualquier signo de infección;
- Latido del corazón rápido, dolor en el pecho.
- Alteraciones en el equilibrio de electrolitos (niveles bajos de potasio, magnesio, fosfato en sangre);
- Dolor abdominal (enterocolitis neutropénica)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Bicnu
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el envase después de “CAD.”. La fecha de caducidad se refiere al último día de ese mes.
Este medicamento será almacenado por su médico o profesional sanitario.
El vial del medicamento en polvo sin abrir debe conservarse en nevera (2°-8°C).
Después de la reconstitución según las recomendaciones, la carmustina inyectable es estable durante 480 horas en refrigeración (2°C -8°C) y 24 horas a temperatura ambiente (25°C ±2°C) en un recipiente de vidrio. Examine los viales reconstituidos para la formación de cristales antes de su uso. Si se observan cristales, se pueden volver a disolver calentando el vial a temperatura ambiente con agitación.
La solución reconstituida diluida hasta 500 ml con cloruro de sodio al 0,9% para inyección o con Dextrosa al 5% para inyección, en recipientes de vidrio o de polipropileno, da como resultado una solución que debe utilizarse dentro de 4 horas a temperatura 25°C ± 2°C y debe estar protegida de la luz. Estas soluciones son también estables hasta 48 horas en nevera (2°C-8° C) protegidas de la luz.
Un indicio de la descomposición es la aparición de una película de aceite en la parte inferior del vial. Si esto ocurre, este medicamento no debe utilizarse más. Cuando usted no esté seguro sobre el hecho de si el producto está enfriado adecuadamente, entonces debe inspeccionar inmediatamente todos y cada uno de los viales de la caja. Para la verificación, mantenga el vial en una luz clara. La carmustina aparece con pequeñas cantidades de escamas secas o masa rígida seca.
Los medicamentos no se deben tirar por los desagües ni a la basura. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Contenido de Bicnu
El principio activo es carmustina.
Un vial de 30 ml contiene 100 mg de carmustina y un vial de 5 ml contiene 3 ml de disolvente esterilizado (propilenglicol).
Resto de los excipientes
Polvo: Sin excipientes
Disolvente: propilenglicol
Aspecto del producto y contenido del envase
Polvo y disolvente para solución para perfusión
Polvo amarillento para la reconstitución.
Aspecto de la solución: incoloro a amarillo claro.
Polvo: vial de vidrio tipo I ámbar (30 ml) sellado con un tapón de goma de bromobutilo gris oscuro y un sellado de aluminio con una tapa de polipropileno.
Disolvente: Vial de vidrio tipo I (5 ml) sellado con un tapón de goma de bromobutilo gris con un sellado de aluminio con una tapa de polipropileno.
Titular de la autorización de comercialización
Laboratorios Tillomed Spain, S.L.U.
C/ Cardenal Marcelo Spínola 8, planta 1ª-puerta F
28016 Madrid
España
Responsable de la fabricación1
SGS Pharma Magyarorszag Kft.
Derkovits Gyula Utca 53,
Budapest XIX, 1193,
Hungría
Tillomed Malta Limited,
Malta Life Sciences Park,
LS2.01.06 Industrial Estate,
San Gwann, SGN 3000, Malta
[1] En el producto comercializado solo se indicará el centro que efectúa realmente la liberación.
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Zentiva Spain S.L.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
España: Bicnu 100 mg Polvo y disolvente para solución para perfusión EFG
Fecha de la última revisión de este prospecto: noviembre 2024.
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/




- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BICNU 100 MG POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION EFGForma farmacéutica: INYECTABLE PERFUSION, 100 mgPrincipio activo: CarmustinaFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 300 mgPrincipio activo: CarmustinaFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 50 mgPrincipio activo: CarmustinaFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para BICNU 100 MG POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BICNU 100 MG POLVO Y DISOLVENTE PARA SOLUCION PARA PERFUSION EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






