
BETOPTIC SUSPENSION 2.5 mg/ml EYE DROPS


How to use BETOPTIC SUSPENSION 2.5 mg/ml EYE DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
BETOPTIC SUSPENSION 2.5 mg/ml eye drops, suspension
Betaxolol
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is BETOPTIC SUSPENSION and what is it used for
- What you need to know before starting to use BETOPTIC SUSPENSION
- How to use BETOPTIC SUSPENSION
- Possible side effects
- Storage of BETOPTIC SUSPENSION
- Package contents and further information
1. What is BETOPTIC SUSPENSION and what is it used for
It is an eye drop that belongs to a group of medications called beta-blockers, which contains betaxolol as the active ingredient. It works by reducing the pressure inside the eye (intraocular pressure), possibly by reducing the production of aqueous humor (fluid in the anterior chamber of the eye).
Betoptic Suspension is indicated for the reduction of elevated intraocular pressure in patients with chronic open-angle glaucoma or ocular hypertension.
It can be used alone or in combination with other medications that reduce intraocular pressure.
2. What you need to know before starting to use BETOPTIC SUSPENSION Do not use Betoptic Suspension
? If you have heart failure, a slow heart rate, or heart rhythm disorders (irregular heartbeats) or other heart disorders that may cause fainting.
? If you have respiratory problems such as severe asthma or severe chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing, and/or long-term coughing).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Betoptic Suspension.
? Only use this medication in your eye(s).
? Before starting to use this medication, inform your doctor if you have or have had:
- coronary artery disease (symptoms may include chest pain or discomfort, shortness of breath, or wheezing), heart failure, low blood pressure.
- first-degree heart block (a condition related to heart rhythm).
- respiratory problems, asthma, or chronic obstructive pulmonary disease.
- poor blood circulation (such as Raynaud's disease, with tingling in the fingers or toes).
- diabetes, as betaxolol may mask the signs and symptoms of low blood sugar levels.
hiperthyroidism, as this medication may mask the signs and symptoms.
- if you have any disease characterized by chronic muscle weakness (such as myasthenia gravis).
? Patients with suspected hyperthyroidism should be carefully monitored.
? If you have a history of severe allergic reactions or atopic reactions (allergy of unknown origin), you may be more sensitive to various allergens. If you experience any severe allergic reaction (skin rash, redness, and itching in the eye, fever, swelling of the throat, tongue, or face) while using this medication, stop treatment immediately and consult your doctor. When receiving any treatment, inform your doctor that you are using this medication.
? If you are being treated with another beta-blocker medication, including another route of administration, you should be cautious.
? Inform your doctor that you are using this medication before undergoing surgery, as betaxolol may alter the effects of some medications used during anesthesia.
? If you have angle-closure glaucoma, you should be prescribed this medication in combination with other medications that act by closing the pupil. You should regularly monitor the eye pressure, especially at the beginning of treatment.
? If you have disorders in the transparent part of the front of the eye (cornea), consult your doctor, as this medication may cause dryness in the eyes.
? If you have skin disorders such as psoriasis, it could worsen.
? If you use contact lenses:
You should not use this medication when wearing contact lenses.
Use in elderly patients
See section 3.
Use in athletes
This medication contains a component, betaxolol, which may produce a positive result in doping tests.
Children
The efficacy and safety have not been established in children.
Other medications and Betoptic Suspension
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
This medication may affect or be affected by other medications you are using, including other eye drops for the treatment of glaucoma. Consult your doctor if you are using or plan to use medications to lower blood pressure, heart medications, or medications to treat diabetes, stimulant medications for the parasympathetic system, and adrenergic psychotropic medications (generally used for attention disorders).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication. Do not use this medication if you are pregnant unless your doctor considers it necessary.
Do not use this medication if you are breastfeeding, as betaxolol may pass into breast milk.
Driving and using machines
You may notice that your vision becomes blurry for a while after applying the eye drops. Do not drive or use machines until this effect has disappeared.
BETOPTIC SUSPENSION contains benzalkonium chloride
This medication contains 0.1 mg of benzalkonium chloride per ml.
Benzalkonium chloride may be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medication and wait 15 minutes before putting them back.
Benzalkonium chloride may cause eye irritation, especially if you have dry eye or other corneal diseases (transparent layer at the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medication.
3. How to use BETOPTIC SUSPENSION
Follow the administration instructions for this medication exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again.
Use in adults
The recommended dose is 1-2 drops in the affected eye(s) twice a day (morning and evening).
It may take several weeks to achieve a stable response to treatment.
Substitution therapy (monotherapy): If you are using another medication for glaucoma and your doctor has indicated that you should switch to Betoptic Suspension, on the first day, continue with the same treatment and also administer 1 drop of Betoptic Suspension in the eye(s) twice a day. The next day, stop the initial medication and continue only with Betoptic Suspension, with the usual dose.
Substitution therapy (multiple therapy): If you are being treated with several medications for glaucoma, your doctor will indicate the dosage schedule, adjusting one medication at a time, with intervals of at least one week.
To achieve greater reduction of intraocular pressure, your ophthalmologist may prescribe other medications that you should use in combination with this medication.
Use in elderly patients
It is recommended to start treatment with the lowest recommended dose of betaxolol, as elderly patients are likely to have age-related conditions, such as heart problems or peripheral vascular diseases.
If the clinical response is not adequate, your doctor may modify the dose to 2 drops of Betoptic Suspension in the affected eye(s) twice a day (morning and evening) with due precautions.
Use in children
The efficacy and safety have not been established in children.
The doctor should carefully weigh the benefits and risks before prescribing this medication to children, performing the necessary tests and history.
In case of administration to children, it is recommended to use the lowest doses once a day. Close monitoring of pediatric patients is required, especially very young children, from 1 or 2 hours after administration of the first dose.
Recommendations for use:
1 2 3 4
- Wash your hands.
- Pick up the bottle (dropper).
- Shake well before use.
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
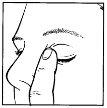
- Hold the bottle, upside down, between your fingers (figure 1).
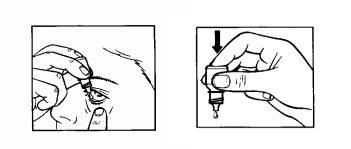
- Tilt your head back. Gently separate the eyelid from the eye with one finger until a pocket forms, where the drop should fall (figure 2).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or surrounding areas, with the dropper. The eye drops could become contaminated.
- Gently squeeze the base of the bottle with your index finger to release one drop at a time (figure 3).
(figure 3).
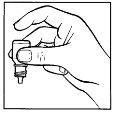
- After using this eye drop, press the edge of the eye next to the nose with your finger for 2 minutes (figure 4). This helps prevent the medication from passing into the rest of the body.
- If you are applying drops in both eyes, repeat all the previous steps with the other eye.
- Close the bottle tightly immediately after using the product.
If a drop falls outside the eye, try again.
If you are using other eye medications, wait at least 5 minutes between the administration of this eye drop and other eye medications. Eye ointments should be administered last.
If you use more Betoptic Suspension than you should
An overdose in the eyes can be eliminated by rinsing the eyes with warm water. Do not apply more drops until it is time for your next dose.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount used.
The symptoms of overdose or in case of accidental ingestion may be: slow heart rate, decreased blood pressure, heart problems or respiratory problems. If this occurs, stop treatment and consult your doctor.
If you forget to use Betoptic Suspension
Do not apply a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember and continue with the next dose that was scheduled. However, if it is almost time for the next dose, do not apply the forgotten dose and continue with the next dose of your regular regimen.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The following side effects have been reported with this medication:
Very common side effects (may affect more than 1 in 10 people):
Eye effects: eye discomfort.
Common side effects (may affect up to 1 in 10 people):
Eye effects: blurred vision, increased tear production.
General effects: headache.
Uncommon side effects (may affect up to 1 in 100 people):
Eye effects: inflammation of the eye with or without damage to the surface (keratitis), conjunctivitis, eyelid inflammation, visual disturbance, sensitivity to light, eye pain, dry eye, tired eyes, eyelid disorder, itching in the eye, eye discharge, eyelid crust, eye inflammation, eye irritation, conjunctival disorder, eye swelling, eye redness.
General effects: decreased heart rate, increased heart rate, asthma, difficulty breathing, nausea, nasal inflammation.
Rare side effects (may affect up to 1 in 1,000 people):
Eye effects: cataract (clouding of the eye).
General effects: fainting, bad taste, coughing, runny nose (rhinorrhea), skin inflammation, rash, decreased blood pressure, anxiety, decreased sexual desire.
During post-marketing experience, additional side effects have been reported, the frequency of which is not known:
Eye effects: eyelid redness.
General effects: irregular heart rhythm, dizziness, hair loss, body weakness, allergy, difficulty sleeping (insomnia), depression.
As with other eye medications, betaxolol is absorbed into the bloodstream. This may cause side effects similar to those seen with intravenous and/or oral beta-blocker medications. The incidence of side effects after eye administration is lower than with medications administered orally or by injection. The adverse reactions described include reactions observed within the class of beta-blocker eye medications when used for the treatment of eye diseases:
- Generalized allergic reactions, including swelling under the skin that may occur in the face and limbs, and may obstruct the airways, causing difficulty swallowing or breathing, urticaria or rash with localized and generalized eruption, itching, severe and sudden allergic reaction.
- Low blood sugar levels.
- Difficulty sleeping, depression, nightmares, memory loss.
- Fainting, fatigue, reduced or absent blood flow to the brain, increased symptoms of myasthenia gravis (muscle disorder), dizziness, unusual sensations such as tingling, headache.
- Eye irritation symptoms (such as burning, stinging, tearing, redness), eyelid inflammation, corneal inflammation, blurred vision, sensitivity of the cornea decreased, dryness in the eyes, corneal erosion, drooping of the upper eyelid (causing the eye to remain half-closed), double vision.
- Chest pain, palpitations, fluid accumulation (edema), changes in heart rate or frequency, other heart disorders, accompanied by difficulty breathing and swelling of the feet and legs due to fluid accumulation (congestive heart failure), cardiac arrest, heart failure.
- Low blood pressure, Raynaud's disease (condition with reduced circulation, causing cold hands and feet).
- Difficulty breathing (mainly in patients with bronchial disease), coughing.
- Digestive disorders such as nausea, indigestion, diarrhea, dry mouth, abdominal pain, etc.
- Hair loss, psoriasiform rash (silvery scales) or worsening of psoriasis, skin rash.
- Muscle pain.
- Sexual dysfunction, decreased libido.
- Fatigue.
Generally, you can continue using the drops unless the side effects are severe. If you are concerned, consult your doctor or pharmacist. Do not stop using this medication without consulting your doctor.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of BETOPTIC SUSPENSION
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the packaging and carton after EXP. The expiration date is the last day of the month indicated.
No special storage conditions are required. Keep the bottle in the outer packaging. To avoid infections, the bottle should be discarded 4 weeks after it was first opened.
Write the date of opening of the bottle in the space provided for this purpose on the carton.
Medications should not be disposed of through wastewater or household waste. Place the packaging and any unused medication in the SIGRE collection point at the pharmacy. If you have any questions, ask your pharmacist how to dispose of the packaging and any unused medication. By doing so, you will help protect the environment.
6. Package contents and further information
Composition of BETOPTIC SUSPENSION
- The active ingredient is betaxolol hydrochloride. Each ml of suspension contains 2.8 mg of betaxolol hydrochloride, equivalent to 2.5 mg/ml of betaxolol (0.25%).
- The other components are: benzalkonium chloride, Amberlite IRP-69 resin (poly (styrene-divinylbenzene) sulfonic acid), carbomer 974P, boric acid, mannitol (E421), disodium edetate, N-laurylsarcosine, hydrochloric acid and/or sodium hydroxide, and purified water.
Appearance of the product and packaging content
Betoptic Suspension is an eye drop suspension; a white or off-white liquid presented in a dropper container (plastic bottle with a cap) in a box.
Each container contains 5 ml of eye drops.
Marketing authorization holder
Immedica Pharma AB
SE-113 63 Stockholm
Sweden
Manufacturer
Siegfried El Masnou, S.A.
C/Camil Fabra, 58
08320 El Masnou – Barcelona, Spain
or
S.A. Alcon-Couvreur N.V. Rijsksweg 14
B-2870 Puurs
Belgium
or
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona, Spain
or
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg, Germany
Date of the last revision of this prospectus:May 2019.
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price3.67 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BETOPTIC SUSPENSION 2.5 mg/ml EYE DROPSDosage form: PROLONGED-RELEASE EYE DROP, 10 mg carteolol hydrochloride/mlActive substance: carteololManufacturer: Bausch + Lomb Ireland LimitedPrescription requiredDosage form: PROLONGED-RELEASE EYE DROP, 20 MG/MLActive substance: carteololManufacturer: Bausch + Lomb Ireland LimitedPrescription requiredDosage form: PROLONGED-RELEASE EYE DROP, 20 mg/ml (i.e. 2% w/v)Active substance: carteololManufacturer: Bausch + Lomb Ireland LimitedPrescription required
Online doctors for BETOPTIC SUSPENSION 2.5 mg/ml EYE DROPS
Discuss questions about BETOPTIC SUSPENSION 2.5 mg/ml EYE DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















