ATROVENT MONODOSIS 250 mcg / 2ml SOLUTION FOR NEBULIZER INHALATION

How to use ATROVENT MONODOSIS 250 mcg / 2ml SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Atrovent Monodosis 250 micrograms/2 ml solution for inhalation by nebulizer
ipratropium bromide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Atrovent Monodosis is and what it is used for
- What you need to know before you use Atrovent Monodosis
- How to use Atrovent Monodosis
- Possible side effects
- Storing Atrovent Monodosis
- Contents of the pack and other information
1. What Atrovent Monodosis is and what it is used for
Atrovent Monodosis belongs to a group of medicines called anticholinergic bronchodilators, which work by relaxing the muscle in the airways, making it easier to breathe.
Atrovent Monodosis 250 micrograms/2 ml solution for inhalation by nebulizer is a medicine used to treat reversible airway obstruction in children aged 6 to 12 years.
Atrovent Monodosis may be given with beta-adrenergic agents (other bronchodilator medicines, such as salbutamol) for the treatment of acute bronchospasm that causes reversible airway obstruction, in cases where treatment with a beta-adrenergic agent does not provide sufficient bronchodilation.
2. What you need to know before you use Atrovent Monodosis
Do not use Atrovent Monodosis:
- If you are allergic (hypersensitive) to ipratropium bromide, to substances similar to ipratropium such as atropine or its derivatives, or to any of the other ingredients listed in section 6.
- In acute episodes of bronchospasm (breathing difficulties) where a rapid response is required.
Warnings and precautions
Talk to your doctor or pharmacist before you start using Atrovent Monodosis.
- If you have a tendency to suffer from narrow-angle glaucoma (increased pressure inside the eye), prostatic hyperplasia (enlargement of the prostate) or urinary outflow obstruction (difficulty urinating).
- Isolated cases of eye complications, such as mydriasis (pupil dilation), narrow-angle glaucoma (increased pressure inside the eye), and eye pain, have occurred when ipratropium bromide, alone or in combination with a beta-2 adrenergic agonist (medicines that dilate the bronchial muscles, such as salbutamol), has entered the eyes due to improper application.
- Eye pain, blurred vision, halos (diffuse lights), or colored images in association with eye redness due to conjunctival congestion and corneal edema (swelling of the cornea) may be signs of increased intraocular pressure (narrow-angle glaucoma). If any combination of these symptoms appears, you should consult your doctor immediately to initiate treatment with a miotic eye drop (which produces pupil contraction and decreases intraocular pressure).
- You should avoid getting the product in your eyes during nebulization, especially in patients who have a tendency to suffer from increased intraocular pressure, so it is recommended to use a mouthpiece or face mask.
- Patients with cystic fibrosis (a disease that affects the mucous and sweat glands, affecting several organs) may be more prone to gastrointestinal motility disorders.
- In exceptional cases, immediate hypersensitivity reactions (rapid allergic reactions) may occur after administration of Atrovent Monodosis, such as urticaria, angioedema (sudden swelling of the skin or mucous membranes), skin rash, bronchospasm (contraction of the bronchial muscles that makes breathing difficult), and oropharyngeal edema (swelling of the mouth and pharynx).
- As with other inhalation medications, Atrovent Monodosis may cause paradoxical bronchospasm, which can be life-threatening. If this occurs, treatment should be stopped immediately and your doctor informed to replace it with an alternative treatment.
Other medicines and Atrovent Monodosis
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Prolonged simultaneous administration of Atrovent Monodosis with other anticholinergic drugs is not recommended.
Beta-adrenergic agents (e.g., salbutamol) and xanthine derivatives (e.g., theophylline) are other bronchodilator medicines and may enhance the bronchodilator effect. Atrovent Monodosis may increase the anticholinergic effects of other medicines.
Atrovent Monodosis can be administered with other commonly used medicines for the treatment of reversible airway obstruction, including beta-adrenergic agents (e.g., salbutamol), methylxanthines (e.g., theophylline), steroids, and sodium cromoglycate, without the appearance of interactions that require dose adjustment.
Simultaneous administration of nebulized ipratropium bromide and beta-mimetics may increase the risk of acute glaucoma in patients with a history of narrow-angle glaucoma (increased intraocular pressure).
Solutions for inhalation of Atrovent Monodosis and sodium cromoglycate that contain benzalkonium chloride as a preservative should not be administered simultaneously in the same nebulizer, due to the risk of precipitation.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Although the preparation is specifically indicated in pediatrics, the usual precautions in pregnancy and breastfeeding are specified below.
Despite the fact that preclinical studies have not shown any risk, safety during pregnancy has not been established. Therefore, the usual precautions when using medicines during this period should be observed, especially during the first three months.
It is not known whether ipratropium bromide can pass into breast milk. However, it is unlikely to be ingested by the infant in significant amounts, especially since the preparation is administered by inhalation. Nevertheless, it should be administered with caution to breastfeeding women.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been conducted. However, it is warned that side effects such as dizziness, eye difficulties in focusing, pupil dilation, and blurred vision may occur during treatment with Atrovent. Therefore, caution is recommended when driving and using machines.
3. How to use Atrovent Monodosis
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Remember to use your medicine.
Your doctor will determine the duration of treatment.
If you think that the effect of Atrovent Monodosis is too strong or too weak, tell your doctor or pharmacist.
The Atrovent Monodosis solution can be inhaled (aspirated) using devices called ultrasonic, electric, manual (e.g., Bird, De Vilbiss, Pari) nebulizers, or with intermittent positive pressure breathing. If you have a wall oxygen supply, the solution should be administered with a flow of 6-8 liters per minute.
It is recommended that the particle size of the nebulized solution be between 1 and 10 microns, although approximately 50% of the total aerosol mass should be contained in particles less than 5 microns.
If necessary, the solution can be diluted with saline solution.
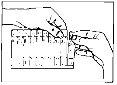
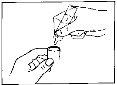
Guidelines for the use of single-dose vials:
Single-dose vials should only be used for inhalation with suitable nebulizers and should not be administered orally.
|
|
Since the single-dose vials do not contain preservatives, it is essential to use their contents immediately after opening the vial to avoid contamination. Partially used, opened, or damaged single-dose vials should be discarded.
The administration of the Atrovent Monodosis solution should be adapted to the individual needs of each patient; patients should be under medical supervision during treatment. It is recommended not to exceed the recommended daily dose for both acute and maintenance treatment. The recommended doses are:
Children from 6 to 12 years:
Maintenance treatment
1 single-dose vial, 3-4 times a day.
Acute attacks
In cases where only a beta-adrenergic agent is used, it may not provide sufficient bronchodilation. In these cases, Atrovent Monodosis can be administered in combination with an inhaled beta-adrenergic agent (e.g., salbutamol), whose dose will be determined by your doctor. The dose of Atrovent Monodosis, in this case, is 1 single-dose vial; repeated doses can be administered until the patient stabilizes.
As a general rule, the recommended daily dose should not be exceeded during treatment. Daily doses of more than 1 mg (more than 4 single-dose vials) should be administered exclusively under medical supervision.
If significant improvement is not achieved with treatment or if the patient's condition worsens, it is necessary to consult your doctor to determine a new treatment. In case of acute dyspnea or rapidly worsening dyspnea (breathing difficulties), you should consult your doctor immediately.
If you use more Atrovent Monodosis than you should
No specific manifestations of overdose have been described. Due to the wide therapeutic margin of Atrovent Monodosis and the inhalation administration of the preparation, it is not expected to appear serious anticholinergic symptoms.
In case of minor anticholinergic symptoms such as dry mouth, visual accommodation disorders (eye problems focusing), and tachycardia (increased heart rate), the treatment to be followed should be to alleviate the symptoms.
If you have used more Atrovent Monodosis than you should, talk to your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Atrovent Monodosis
Do not use a double dose to make up for forgotten doses.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects (at least 1 in 100 patients) are headache, dizziness, cough, throat irritation, nausea, dry mouth, gastrointestinal motility disorders (e.g., change in bowel habits, gastroesophageal reflux, dyspepsia).
Uncommon side effects (at least 1 in 1,000 patients) are hypersensitivity, anaphylactic reaction (severe allergic reaction), blurred vision, mydriasis (pupil dilation), increased intraocular pressure, halos (diffuse lights), or colored images associated with eye redness (glaucoma), eye pain, halos (diffuse lights), eye redness, corneal edema (swelling of the cornea), palpitations, supraventricular tachycardia, constipation, diarrhea, vomiting, stomatitis (inflammation of the mouth), oral edema (swelling of the mouth), rash, pruritus (itching), angioedema (swelling of the face, lips, mouth, tongue, or throat that can cause difficulty swallowing or breathing), and oropharyngeal edema (swelling of the mouth and pharynx).
Rare side effects (at least 1 in 10,000 patients) are bronchospasm (chest tightness, wheezing, or shortness of breath), paradoxical bronchospasm (narrowing of the bronchial walls due to the inhalation itself), laryngeal contraction, pharyngeal edema (swelling of the throat), throat dryness, visual accommodation disorder (eye difficulty focusing), urticaria, increased heart rate, and atrial fibrillation.
Very rare side effects (less than 1 in 10,000 patients) are tremors, metallic or unpleasant taste, nasal congestion, insomnia, unusual fatigue or weakness, and hypotension.
If you experience any side effect, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Atrovent Monodosis
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton. The expiry date refers to the last day of the month shown.
Do not store above 30°C. Store in the original package.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Atrovent Monodosis
- The active substance is ipratropium bromide. Each 2 ml single-dose vial contains 261 micrograms of ipratropium bromide monohydrate (equivalent to 250 micrograms of anhydrous ipratropium bromide).
- The other ingredients are sodium chloride, hydrochloric acid, and purified water.
Appearance and packaging
Atrovent Monodosis 250 micrograms/2 ml is a solution for inhalation by nebulizer, presented in boxes of 20 single-dose vials with 2 ml of solution for inhalation by nebulizer and in a clinical pack with 100 single-dose vials of 2 ml of solution for nebulizer.
Marketing authorization holder
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
Spain
Manufacturer
Laboratoire Unither
Zone Industrialle de Longpré
10 rue Andre Durouchez
80084 AMIENS Cedex 2
France
This leaflet was approved in April 2019
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ATROVENT MONODOSIS 250 mcg / 2ml SOLUTION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 0.0040 g/aerosolActive substance: ipratropium bromideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 20 µgActive substance: ipratropium bromideManufacturer: Boehringer Ingelheim Espana S.A.Prescription requiredDosage form: PULMONARY INHALATION, 500 µgActive substance: ipratropium bromideManufacturer: Boehringer Ingelheim Espana S.A.Prescription required
Online doctors for ATROVENT MONODOSIS 250 mcg / 2ml SOLUTION FOR NEBULIZER INHALATION
Discuss questions about ATROVENT MONODOSIS 250 mcg / 2ml SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions