ASPAVELI 1080 MG SOLUCION PARA PERFUSION

Cómo usar ASPAVELI 1080 MG SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ASPAVELI 1 080 mg solución para perfusión
pegcetacoplán
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es ASPAVELI y para qué se utiliza
- Qué necesita saber antes de empezar a usar ASPAVELI
- Cómo usar ASPAVELI
- Posibles efectos adversos
- Conservación de ASPAVELI
- Contenido del envase e información adicional
1. Qué es ASPAVELI y para qué se utiliza
Qué es ASPAVELI
ASPAVELI es un medicamento que contiene el principio activo pegcetacoplán. Pegcetacoplán ha sido diseñado para unirse a la proteína C3 del complemento, la cual forma parte del sistema de defensa del organismo denominado “sistema del complemento”. Pegcetacoplán impide que el sistema inmunitario de su organismo destruya los glóbulos rojos.
Para qué se utiliza ASPAVELI
ASPAVELI se utiliza para el tratamiento de pacientes adultos con una enfermedad denominada hemoglobinuria paroxística nocturna (HPN) que tienen anemia como consecuencia de esta enfermedad.
En los pacientes con HPN, el “sistema del complemento” es hiperactivo y ataca a sus glóbulos rojos, lo que puede provocar recuentos sanguíneos bajos (anemia), cansancio, dificultad para funcionar, dolor, dolor abdominal, orina oscura, falta de aliento, dificultad para tragar, disfunción eréctil y coágulos en la sangre. Al unirse a la proteína C3 y bloquearla, este medicamento puede impedir que el sistema del complemento ataque a los glóbulos rojos, controlando así los síntomas de la enfermedad. Se ha demostrado que este medicamento aumenta el número de glóbulos rojos (disminuye la anemia), lo que puede mejorar estos síntomas.
2. Qué necesita saber antes de empezar a usar ASPAVELI
No use ASPAVELI
- si es alérgico al pegcetacoplán o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene una infección causada por las denominadas bacterias encapsuladas.
- si no está vacunado contra Neisseria meningitidis, Streptococcus pneumoniaey Haemophilus influenzae.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar ASPAVELI.
Síntomas de las infecciones
Antes de empezar a usar ASPAVELI, informe a su médico si tiene alguna infección.
Dado que el medicamento se dirige al sistema del complemento, que forma parte de las defensas del organismo contra las infecciones, el uso de este medicamento aumenta el riesgo de padecer infecciones, incluidas las causadas por las denominadas bacterias encapsuladas, como Streptococcus pneumoniae, Neisseria meningitidisy Haemophilus influenzae. Se trata de infecciones graves que afectan a la nariz, la garganta y los pulmones o al tejido que recubre el cerebro y que pueden diseminarse por toda la sangre y el cuerpo.
Consulte a su médico antes de empezar a usar ASPAVELI para asegurarse de recibir las vacunas contra Streptococcus pneumoniae, Neisseria meningitidisy Haemophilus influenzaesi no ha recibido antes estas vacunas.Si ya ha recibido estas vacunas en el pasado, aún podría necesitar vacunas adicionales antes de empezar a usar este medicamento. Estas vacunas deben administrarse al menos 2 semanas antes de comenzar el tratamiento. Si no puede vacunarse con 2 semanas de antelación, su médico le recetará antibióticos para reducir el riesgo de infección durante 2 semanas después de haber sido vacunado. Tras la vacunación, es posible que su médico le vigile más estrechamente para detectar síntomas de infección.
Síntomas de infección
Si experimenta alguno de los siguientes síntomas, debe informar inmediatamente a su médico:
- dolor de cabeza y fiebre
- fiebre y sarpullido
- fiebre con o sin escalofríos o temblores
- falta de aliento
- pulso elevado
- piel sudorosa
- dolor de cabeza con rigidez de cuello o espalda
- dolor de cabeza con náuseas (ganas de vomitar) o vómitos
- ojos sensibles a la luz
- dolores musculares con síntomas parecidos a la gripe
- confusión
- dolor o malestar intenso
Asegúrese de mantener sus vacunas al día. También debe ser consciente de que las vacunas reducen el riesgo de padecer infecciones graves, pero no previenen todas las infecciones graves. De acuerdo con las recomendaciones nacionales, su médico podría considerar que necesita medidas complementarias, como medicamentos antibacterianos, para prevenir las infecciones.
Reacciones alérgicas
En algunos pacientes pueden aparecer reacciones alérgicas. En caso de reacción alérgica grave, suspenda la perfusión de ASPAVELI y solicite asistencia médica inmediata. La reacción alérgica grave puede manifestarse como dificultad para respirar, dolor u opresión en el pecho y/o sensación de mareo/desvanecimiento, picor cutáneo intenso o bultos elevados en la piel, hinchazón de la cara, los labios, la lengua y/o la garganta, que puede causar dificultad para tragar o síncope.
Reacciones en el lugar de la inyección
Se han observado reacciones en el lugar de la inyección con el uso de ASPAVELI. Antes de la autoadministración, debe recibir una formación adecuada sobre la técnica de inyección correcta.
Controles analíticos
Durante el tratamiento con ASPAVELI, su médico le realizará controles periódicos, incluidos análisis de las concentraciones de lactato deshidrogenasa (LDH) en sangre y análisis de la función renal, y es posible que le ajuste la dosis, si es necesario.
Efectos sobre las pruebas analíticas
Se debe evitar el uso de reactivos de sílice en las pruebas de coagulación, ya que puede provocar una prolongación artificial del tiempo de tromboplastina parcial activado (TTPa).
Niños y adolescentes
No administre este medicamento a niños menores de 18 años, ya que no se dispone de datos sobre su seguridad y eficacia en este grupo.
Otros medicamentos y ASPAVELI
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Mujeres en edad fértil
No se conocen los efectos del medicamento en el feto. Se recomienda utilizar métodos anticonceptivos efectivos durante el tratamiento y hasta 8 semanas después del mismo en las mujeres que pueden quedarse embarazadas. Consulte a su médico antes de utilizar este medicamento.
Embarazo/lactancia
No se recomienda utilizar ASPAVELI durante el embarazo y la lactancia. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
ASPAVELI contiene sorbitol
El sorbitol es una fuente de fructosa. Si su médico le ha indicado que usted padece una intolerancia a ciertos azúcares, o se le ha diagnosticado intolerancia hereditaria a la fructosa (IHF), una enfermedad genética rara, en la que el paciente no puede descomponer la fructosa, consulte usted con su médico antes de tomar este medicamento.
ASPAVELI contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar ASPAVELI
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Al menos 2 semanas antes de comenzar el tratamiento con este medicamento, su médico revisará su historia clínica y puede que le administre una o más vacunas. Si no puede vacunarse al menos 2 semanas antes de empezar el tratamiento con ASPAVELI, para reducir el riesgo de infección, su médico le recetará antibióticos durante 2 semanas después de haberse vacunado.
Dosis
La dosis inicial recomendada para los adultos con HPN es de 1 080 mg dos veces por semana. Debe tomar la dosis dos veces por semana, el Día 1 y el Día 4 de cada semana de tratamiento.
Si va a reemplazar otro tipo de medicamento para la HPN, denominado inhibidor de C5, por ASPAVELI, debe tomar ASPAVELI además de su dosis actual del inhibidor de C5 según se le haya prescrito durante 4 semanas. Después de 4 semanas, debe dejar de tomar el inhibidor de C5.
La dosis o el intervalo de dosificación no se deben modificar sin consultar a su médico. Su médico puede ajustar su dosis a 1 080 mg cada tres días (p. ej., Día 1, Día 4, Día 7, Día 10, Día 13 y así sucesivamente) si procede. Si cree que ha olvidado una dosis, hable con su médico lo antes posible.
Forma y vía de administración
ASPAVELI está previsto para ser administrado en forma de perfusión (gotero) bajo la piel utilizando una bomba de perfusión. Las primeras dosis del medicamento se las administrará un profesional sanitario en una clínica o centro de tratamiento. Si el tratamiento funciona bien, su médico puede comentarle la posibilidad de administrarse el medicamento en casa. En caso de que esto sea adecuado, un profesional sanitario le enseñará a usted o a un cuidador cómo administrar la perfusión.
Velocidad(es) de perfusión
El tiempo de perfusión habitual es de unos 30 minutos si se utilizan dos lugares de perfusión o de unos 60 minutos si se utiliza uno solo. La perfusión debe iniciarse sin demora (y completarse dentro de las 2 horas siguientes a la preparación de la jeringa) después de cargar este medicamento en la jeringa
Instrucciones de uso
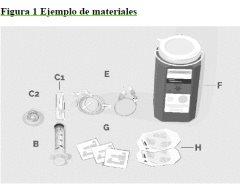
Paso 1 | Prepárese para la perfusión Antes de comenzar:
C1 Aguja de transferencia O BIEN C2 Dispositivo de transferencia sin aguja para extraer el producto del vial
|
|
Limpie a fondo la superficie de trabajo con una toallita con alcohol. | ||
Lávese bien las manos con agua y jabón. Séquese las manos. | ||
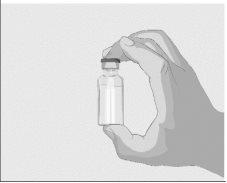
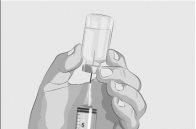
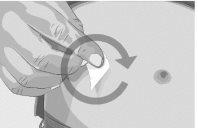
Paso 2 | Compruebe el vial y el líquido Saque el vial de la caja. Examine cuidadosamente el líquido del vial. ASPAVELI es un líquido transparente, entre incoloro y ligeramente amarillento. Compruebe si hay partículas o cambios de color (Figura 2). No utilice el vial si:
| Figura 2
|
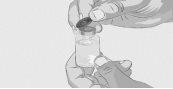
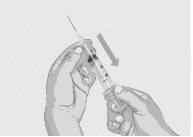
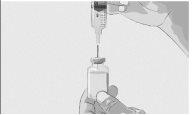
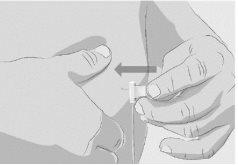
Paso 3 | Prepare y llene la jeringa Retire la cápsula de cierre desprendible protectora del vial para descubrir la parte central del tapón de goma gris del vial (Figura 3). Tire la cápsula de cierre. Limpie el tapón con una toallita con alcohol nueva y deje que se seque. Opción 1: Si se utiliza un dispositivo de transferencia sin aguja (como un adaptador de vial), siga las instrucciones proporcionadas por el fabricante del mismo. O BIEN Opción 2: Si la transferencia se realiza con una aguja de transferencia y una jeringa, siga las siguientes instrucciones:
| Figura 3
Figura 4
Figura 5
Figura 6
Figura 7
|
Paso 4 | Prepare el sistema de bomba de perfusión de jeringa y los tubos Reúna los componentes de la bomba de perfusión y siga las instrucciones del fabricante del dispositivo para preparar la bomba y los tubos. | |
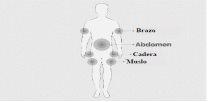
Paso 5 | Prepare el lugar o lugares de perfusión
| Figura 8
Figura 9
Figura 10
|
Paso 6 | Inserte y asegure la aguja o agujas de perfusión
| Figura 11
Figura 12
|
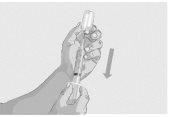
Paso 7 | Inicie la perfusión Siga las instrucciones del fabricante del dispositivo para iniciar la perfusión. Inicie la perfusión sin demora después de extraer la solución a la jeringa. | |
Paso 8 | Complete la perfusión Siga las instrucciones del fabricante del dispositivo para completar la perfusión. | |
Paso 9 | Registre la perfusión Registre su tratamiento según las indicaciones de su profesional sanitario. | |
Paso 10 | Recoja
| Figura 13
|
Si olvidó usar ASPAVELI
Si olvida una dosis, deberá recibirla tan pronto como sea posible; luego reciba la siguiente dosis a la hora prevista.
Si interrumpe el tratamiento con ASPAVELI
La HPN es una enfermedad que dura toda la vida, por lo que se espera que utilice este medicamento durante mucho tiempo. Si desea dejar de utilizar el medicamento, consulte primero con su médico. Si interrumpe el tratamiento de forma repentina, puede correr el riesgo de que los síntomas empeoren.
Si su médico decide interrumpir el tratamiento con este medicamento, siga sus instrucciones sobre cómo interrumpirlo. Su médico le vigilará estrechamente durante al menos 8 semanas después de interrumpir el tratamiento para detectar cualquier signo de destrucción de glóbulos rojos (hemólisis) debido a la HPN. Los síntomas o problemas que pueden ocurrir por la destrucción de los glóbulos rojos incluyen:
- cansancio
- falta de aliento
- sangre en la orina
- dolor en la zona del estómago (abdomen)
- disminución del número de glóbulos rojos
- coágulos en la sangre (trombosis)
- dificultad para tragar
- disfunción eréctil en los hombres
Póngase en contacto con su médico si presenta cualquiera de estos signos y síntomas.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Su médico hablará con usted sobre los posibles efectos adversos y le explicará los riesgos y beneficios de ASPAVELI antes del tratamiento.
El efecto adverso más grave es una infección grave.
Si experimenta alguno de los síntomas de infección (ver sección 2 “Síntomas de infección"), debe informar inmediatamente a su médico.
En caso de duda sobre qué son los siguientes efectos adversos, pida a su médico que se los explique.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Reacciones en el lugar de la inyección: estas incluyen enrojecimiento (eritema), hinchazón, picor (prurito), cardenales y dolor. Estas reacciones suelen desaparecer en pocos días
- Infección de la nariz, la garganta o las vías respiratorias (infección de las vías respiratorias altas)
- Diarrea
- Destrucción de los glóbulos rojos (hemólisis)
- Dolor de estómago (dolor abdominal)
- Dolor de cabeza
- Cansancio (fatiga)
- Fiebre o temperatura elevada (pirexia)
- Tos
- Infección de las vías urinarias
- Complicaciones relacionadas con las vacunaciones obligatorias
- Dolor en los brazos y las piernas (dolor en las extremidades)
- Mareo
- Dolor en las articulaciones (artralgia)
- Dolor de espalda
Frecuentes(pueden afectar hasta a 1 de cada 10 personas):
- Reacción en el lugar de la inyección, como enrojecimiento o endurecimiento de la piel
- Infección del oído, la boca o la piel
- Dolor de garganta
- Menor número de plaquetas en la sangre (trombocitopenia), que puede hacer que se sangre o se formen cardenales con mayor facilidad de lo normal
- Náuseas (ganas de vomitar)
- Niveles disminuidos de potasio en la sangre (hipopotasemia)
- Sangrado nasal (epistaxis)
- Enrojecimiento de la piel (eritema)
- Dolor en los músculos (mialgia)
- Infección del estómago y los intestinos, que puede causar síntomas leves a graves de náuseas, vómitos, retortijones, diarrea (infección gastrointestinal)
- Pruebas hepáticas elevadas
- Dificultad para respirar (disnea)
- Menor número de glóbulos blancos (neutropenia)
- Deterioro de la función renal
- Orina de color diferente
- Presión arterial alta
- Espasmos musculares
- Nariz taponada (congestión nasal)
- Sarpullido
- Infección en la sangre (sepsis)
- Infección vírica
- Infección por hongos
- Infección de las vías respiratorias
- Infección ocular
- COVID-19
- Infección bacteriana
- Infección vaginal
Poco frecuentes(pueden afectar hasta a 1 de cada 100 personas):
- Inflamación del cuello uterino
- Infección en la ingle
- Bolsa de pus en la nariz (absceso nasal)
- Neumonía
- Tuberculosis
- Infección esofágica por levaduras
- Bolsa de pues en el ano (absceso anal)
- Ronchas
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ASPAVELI
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la caja después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 ºC y 8 ºC).
- Conservar el vial en la caja original para protegerlo de la luz.
- Los medicamentos no se deben tirar por los desagües. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ASPAVELI
El principio activo es pegcetacoplán 1 080 mg (54 mg/ml en un vial de 20 ml).
Los demás componentes son sorbitol (E 420) (ver sección 2 “ASPAVELI contiene sorbitol”), ácido acético glacial, acetato de sodio trihidratado (ver sección 2 “ASPAVELI contiene sodio”), hidróxido de sodio (ver sección 2 “ASPAVELI contiene sodio”) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
ASPAVELI es una solución para perfusión subcutánea transparente, entre incolora y ligeramente amarillenta (54 mg/ml en cada vial de 20 ml). Las soluciones turbias o con partículas o cambios de color no deben utilizarse.
Tamaños de envases
ASPAVELI se presenta en un envase de 1 vial o en un multienvase de 1 x 8 viales.
Tenga en cuenta que el envase no contiene torundas con alcohol, agujas ni otros suministros o equipos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm
Suecia
Responsable de la fabricación
Swedish Orphan Biovitrum AB (publ)
Norra Stationsgatan 93
113 64 Stockholm
Suecia
Fecha de la última revisión de este prospecto: 05/2024.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ASPAVELI 1080 MG SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 300 MGPrincipio activo: EculizumabFabricante: Amgen Technology (Ireland) Unlimited CompanyRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 300 mgPrincipio activo: EculizumabFabricante: Samsung Bioepis Nl B.V.Requiere recetaForma farmacéutica: CÁPSULA, 200 mgPrincipio activo: iptacopanFabricante: Novartis Europharm LimitedRequiere receta
Médicos online para ASPAVELI 1080 MG SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ASPAVELI 1080 MG SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes