
AMICACINA BRAUN 500 MG/2ML SOLUCIÓN INYECTABLE

Cómo usar AMICACINA BRAUN 500 MG/2ML SOLUCIÓN INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Amicacina Braun 500 mg/2ml solución inyectable
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento
|
Contenido del prospecto:
- Qué es Amicacina Braun 500 mg/2ml y para qué se utiliza
- Qué necesita saber antes de empezar a usar Amicacina Braun 500 mg/2ml
- Cómo usar Amicacina Braun 500 mg/2ml
- Posibles efectos adversos
- Conservación de Amicacina Braun 500 mg/2ml
- Contenido del envase e información adicional
1. Qué es Amicacina Braun 500 mg/2ml y para qué se utiliza
El medicamento Amicacina Braun 500 mg/2ml es una solución acuosa de amikacina, antibiótico bactericida del grupo de los aminoglucósidos.
Los antibióticos se utilizan para tratar infecciones bacterianas y no sirven para tratar infecciones víricas como la gripe o el catarro.
Es importante que siga las instrucciones relativas a la dosis, las tomas y la duración del tratamiento indicadas por su médico.
No guarde ni reutilice este medicamento. Si una vez finalizado el tratamiento le sobra antibiótico, devuélvalo a la farmacia para su correcta eliminación. No debe tirar los medicamentos por el desagüe ni a la basura.
Se utiliza para el tratamiento, a corto plazo, de las infecciones graves producidas por microorganismos sensibles. Se utiliza principalmente en los casos siguientes:
- infección en sangre, que se conoce con el nombre de sepsis
- infecciones graves de las vías respiratorias inferiores
- infecciones del sistema nervioso central (incluyendo meningitis)
- infecciones intraabdominales, incluyendo peritonitis
- infecciones de la piel, huesos y tejidos blandos (incluyendo quemaduras)
- infecciones complicadas y recidivantes de las vías urinarias
- infecciones después de una intervención quirúrgica
2. Qué necesita saber antes de empezar a usar Amicacina Braun 500 mg/2ml
No use Amicacina Braun 500 mg/2ml:
- Si es alérgico a la amikacina o a otros antibióticos aminoglucósidos,
- Si padece miastenia gravis,
- No debe administrarse con otros productos que sean tóxicos para el oído o el riñón, ni con diuréticos potentes,
Advertencias y precauciones:
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Amicacina Braun 500 mg/2ml.
Informe a su médico de cualquier alergia o problema médico que tenga o haya tenido, especialmente:
- si es alérgico a la amikacina o a otros antibióticos aminoglucósidos,
- si sus riñones no funcionan bien, ya que puede aumentar el riesgo de toxicidad,
- si tiene trastornos musculares, como miastenia graviso Parkinson, ya que se puede agravar la debilidad muscular,
- si está tomando diuréticos que puedan ser tóxicos para el oído,
- si aparecen síntomas de toxicidad para los oídos tales como mareo, vértigo, tinnitus (sensación de campanilleo), zumbidos en los oídos y pérdida de audición o síntomas de toxicidad en los riñones.
- si aparece diarrea intensa,
- es posible que su infección no responda a la amikacina si no respondió a otros aminoglucósidos, y puede tener una reacción alérgica si ya es alérgico a otro aminoglucósido,
- si usted o los miembros de su familia tienen una enfermedad por mutación mitocondrial (una enfermedad genética) o pérdida de audición debida a los antibióticos, se le aconseja que informe a su médico o farmacéutico antes de tomar un aminoglucósido; ciertas mutaciones mitocondriales pueden aumentar su riesgo de pérdida auditiva con este medicamento. Su médico puede recomendarle pruebas genéticas antes de la administración de Amicacina Braun 500 mg/2ml,
- amikacina puede producir alteraciones en los valores de análisis de algunas sustancias como: nitrógeno ureico, transaminasas, fosfatasa alcalina, bilirrubina, creatinina, lactato deshidrogenasa, sodio, potasio, magnesio y calcio.
Para reducir el riesgo de daño nervioso del oído y del riñón, su médico será especialmente cuidadoso al evaluar lo siguiente:
- Supervisión de la audición, el equilibrio y la función renal antes, durante y después del tratamiento.
- Posología estrictamente según la función renal.
- Si padece función renal alterada, se tendrán en cuenta para la dosis total los antibióticos administrados de manera adicional directamente en el lugar de la infección.
- Supervisión de las concentraciones séricas de amikacina durante el tratamiento si los pormenores de su caso lo exigen.
- Si ya padece daño nervioso en el oído (deterioro de la función auditiva o del equilibrio), o si el tratamiento es a largo plazo, se requiere un control adicional de la función del equilibrio y la audición.
- En caso posible, recibirá el tratamiento con amikacina durante no más de 10-14 días (normalmente, 7-10 días).
- Debe transcurrir tiempo suficiente, 7-14 días, entre los tratamientos individuales con amikacina y otros antibióticos estrechamente relacionados.
- Evitar la administración de otras sustancias con posibles efectos dañinos sobre el nervio auditivo o los riñones en combinación con amikacina. Si esto resulta inevitable, se requiere un control especialmente cuidadoso de la función renal.
- El nivel de fluidos corporales y la producción de orina deben estar en el rango normal.
Niños
Este medicamento se administrará con precaución y solamente si no existe otra alternativa en pacientes prematuros y recién nacido debido al desarrollo renal incompleto de estos pacientes.
Otros medicamentos y Amicacina Braun 500 mg/2ml
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
La administración de Amicacina Braun 500 mg/2ml junto con los siguientes medicamentos puede hacer necesario modificar la dosis de alguno de ellos o la interrupción del tratamiento.
- Otros antibióticos aminoglucósidos (gentamicina, tobramicina…) o capreomicina.
- Amfotericina, vancomicina, agentes que disminuyen la inmunidad, agentes citotóxicos (tóxicos para las células como ciclosporina o cisplatino), cefalosporinas (cefalotina) o diuréticos potentes.
- Anestésicos hidrocarburos halogenados por inhalación, transfusiones masivas de sangre citratada y bloqueantes neuromusculares.
- Antihistamínicos, buclizina, ciclizina, loxapina, meclozina, fenotiazinas, tioxantenos o trimetobenzamida.
- Antiemiasténicos (medicamentos para tratar la miastenia grave - debilidad de los músculos).
- Indometacina.
- Malation.
- Antibióticos polipeptídicos (colistina, polimixina).
- Analgésicos opiáceos.
- Antibióticos betalactámicos (penicilina).
- Indometacina intravenosa en niños.
Embarazo y lactancia:
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No está recomendado el tratamiento durante el embarazo, aunque el médico valorará la conveniencia de su utilización. Si el medicamento se utiliza durante el embarazo, o si la paciente se queda embarazada durante el tratamiento, debe ser informada de los posibles riesgos.
No se tienen datos sobre la excreción por la leche materna, pero como regla general no debe realizarse la lactancia si la madre se encuentra bajo tratamiento.
Conducción y uso de máquinas:
No existe evidencia de efectos sobre la capacidad para conducir vehículos o utilizar maquinaria. Sin embargo, dicha capacidad puede verse alterada si aparecen reacciones adversas como mareo, vértigo, y letargia.
Amicacina Braun 500 mg/2ml contiene metabisulfito de sodio (E-223), metilparabeno (E-218) y propilparabeno (E-216).
Este medicamento puede producir reacciones alérgicas graves y broncoespasmo (sensación repentina de ahogo) porque contiene metabisulfito de sodio (E-223).
Puede producir reacciones alérgicas (posiblemente retardadas) y, excepcionalmente, broncoespasmo (sensación repentina de ahogo) porque contiene metilparabeno (E-218) y propilparabeno (E-216).
Este medicamento contiene menos de 23 mg de sodio (1mmol) por cada vial de 2 ml; esto es, esencialmente “exento de sodio”.
3. Cómo usar Amicacina Braun 500 mg/2ml
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico, farmacéutico o enfermero. En caso de duda pregunte a su médico, farmacéutico o enfermero.
Su médico le indicará la duración de su tratamiento. No suspenda el tratamiento antes.
Su médico determinará cual es la dosis más adecuada para usted, según su edad, peso, estado general, gravedad de la infección y funcionamiento de los riñones. Debe realizarse un seguimiento de la función renal durante el tratamiento.
Deben evitarse concentraciones máximas (30-90 minutos después de la inyección) superiores a 35 microgramos/min y concentraciones mínimas (justo antes de la siguiente dosis) superiores a 10 microgramos/min.
Si la función renal es normal, la dosis recomendada para adultos es de 15-20 mg/kg/día dividida en dosis única diaria o repartida en 2 o 3 dosis iguales administradas a intervalos equivalentes.
Uso en niños
Adolescentes (de 12 a menos de 18 años) y niños (de 2 a 11 años):
La posología recomendada es igual a la del adulto.
Recién nacidos a términos de 2 semanas o más y lactantes (de 28 días a 23 meses):
La dosis recomendada en niños mayores de 2 semanas es de 7,5 mg/kg cada 12 h o 5 mg/kg cada 8 h.
Recién nacidos a término de menos de 2 semanas (0 a 13 días):
Se administrará una primera dosis de carga de 10 mg/kg, para seguir con 7,5 mg/kg cada 12 horas.
Prematuros:
La dosis recomendada es de 7,5 mg/kg cada 12 horas.
Amicacina Braun 500 mg/2ml puede no ser adecuada para ajustar la posología en todos los casos. Existen otras presentaciones de solución para perfusión intravenosa que se puede utilizar en esta situación.
Si el paciente tiene alterada la función renal, el médico la controlará cuidadosamente y si la situación lo requiere se modificará la dosis o se ampliarán los intervalos entre dosis.
Amikacina Braun 500 mg/2 ml debe administrarse por vía intramuscular.
Si usa más Amicacina Braun 500 mg/2ml de la que debiera:
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o consulte al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida. Se recomienda llevar el envase y el prospecto del medicamento al profesional sanitario.
En caso de presentarse una reacción tóxica por una elevada dosificación o acumulación, a tener en cuenta especialmente en pacientes con insuficiencia renal grave, la diálisis peritoneal o hemodiálisis pueden favorecer la eliminación del antibiótico.
Si se produjese una reacción de hipersensibilidad se suspenderá su administración, aplicándose al paciente el tratamiento específico adecuado a la naturaleza e intensidad de la misma (antihistamínicos, corticosteroides, adrenalina...)
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Amicacina Braun 500 mg puede producir efectos adversos, aunque no todas las personas los sufran.
A continuación, se presenta el listado de reacciones adversas en función de los sistemas afectados y en orden decreciente de frecuencia según los criterios:
Muy frecuentes (> 1/10), frecuentes (>1/100, < 1/10), poco frecuentes (>1/1000, <1/100), raras (>1/10000, < 1/1000) y muy raras (<1/10000)
Trastornos renales y urinarios: | muy frecuentes: toxicidad renal: aumento del nitrógeno ureico y no proteico y de la creatinina en sangre, albuminuria, presencia en la orina de glóbulos rojos y blancos… |
Trastornos del oído y del laberinto: | muy frecuentes: toxicidad del sistema nervioso y del oído: pérdida de la audición, vértigo, daño coclear incluyendo pérdida de la audición en frecuencias altas. Pueden producirse mareos, ataxia (enfermedad que afecta a los movimientos voluntarios) vértigo, zumbido de oídos y pérdida de audición. |
Trastornos del sistema nervioso: | muy frecuentes: toxicidad del sistema nervioso y-bloqueo neuromuscular: parálisis muscular aguda y apnea (suspensión de la respiración), entumecimiento, hormigueo, espasmos musculares y convulsiones. poco frecuentes: dolor de cabeza, temblores. |
Trastornos de la piel y del tejido subcutáneo: | poco frecuentes: erupción en la piel, enrojecimiento y elevación de la temperatura en el lugar de inyección. |
Trastornos gastrointestinales: | poco frecuentes: náuseas, vómitos. |
Trastornos musculoesqueléticos y del tejido conjuntivo: | poco frecuentes: parestesia (sensación de adormecimiento, hormigueo, o ardor en la piel), artralgia (dolor en articulaciones). |
Trastornos generales y alteraciones en el lugar de la administración: | poco frecuentes: dolor en el sitio de la inyección. |
Trastornos de la sangre y del sistema linfático: | raras: eosinofilia, anemia (baja concentración de glóbulos rojos). |
Trastornos cardíacos: | raras: hipotensión (baja tensión arterial); hipomagnesemia (bajo nivel de magnesio). |
Notificación de sospechas de reacciones adversas
Es importante notificar sospechas de reacciones adversas al medicamento tras su autorización. Ello permite una supervisión continuada de la relación beneficio/riesgo del medicamento. Se invita a los profesionales sanitarios a notificar las sospechas de reacciones adversas a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es
5. Conservación de Amicacina Braun 500 mg/2ml.
Mantener fuera de la vista y del alcance de los niños.
Conservar en un sitio fresco y en el embalaje original para protegerlo de la luz.
No utilice Amicacina Braun 500 mg/2ml después de la fecha de caducidad que aparece en el envase. La fecha de caducidad es el último día del mes que se indica.
El contenido de los viales debe ser utilizado inmediatamente tras su apertura. Una vez abierto el envase, desechar la porción no utilizada de la solución.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deAmicacina Braun 500 mg/2ml
El principio activo de Amicacina Braun 500 mg/2ml es amikacina sulfato
Cada vial contiene 500 mg de amikacina base.
Los demás componentes son: metilparabeno (E-218), propilparabeno (E-216), metabisulfito de sodio (E-223), edetato disódico, y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Se presenta en envases conteniendo 1 y 50 viales de vidrio de 2 ml.
Titular de la autorización de comercialización y responsable de la fabricación
- Braun Medical, S.A.
Ctra. de Terrassa, 121
08191-Rubí (Barcelona)
España
Fecha de la última revisión de este prospecto:junio 2023
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
----------------------------------------------------------------------------------------------
Esta información está destinada únicamente a médicos o profesionales del sector sanitario:
Deben observarse cuidadosamente las funciones tanto renal como del nervio auditivo en paciente con insuficiencia renal, cuando se use durante periodos largos o cuando se administre en dosis superiores a las recomendadas ya que han sido comunicados trastornos del VIII par craneal, así como de la función renal. La aparición de signos de nefro u ototoxicidad determinará un reajuste de la dosificación o la suspensión del tratamiento según los casos.
Deben estudiarse los niveles plasmáticos de amikacina, ajustando su dosificación para evitar prolongados niveles superiores a 35 microgramos/ml. Se debe examinar la orina para detectar incrementos en la excreción de proteínas, la presencia de células o cilindros y disminución de la densidad.
La ototoxicidad en niños no está bien determinada.
En caso de aparecer sobreinfecciones producidas por microorganismos resistentes, debe suspenderse el tratamiento y aplicarse la terapia adecuada.
Los pacientes deben estar bien hidratados durante el tratamiento.
Posología
Los pacientes con función renal normal pueden recibir una dosis única diaria, siempre que el máximo de concentración no exceda de 35 mg/ml. La duración usual del tratamiento es de 7 a 10 días.
En infecciones difíciles y complicadas puede ser necesario un tratamiento más prolongado. En estos casos, se vigilarán las funciones renales, auditiva (coclear) y vestibular.
La dosis recomendada para adultos, adolescentes y niños de más de 2 años es de 15-20 mg/kg/día en forma de dosis única diaria o repartida en dos o tres dosis iguales administradas a intervalos equivalentes (es decir, para una dosis de 15 mg/kg/día, se administra repartida en dosis de 7,5 mg/kg cada 12 horas o 5 mg/kg cada 8 horas).
La dosis total no debe sobrepasar 1,5 g/día.
Población pediátrica
Recién nacidos a término de 2 semanas o más y lactantes (28 días a 23 meses):
La dosis recomendada en niños mayores de 2 semanas es de 7,5 mg/kg cada 12 h o 5 mg/kg cada 8 h.
Recién nacidos a término de menos de 2 semanas (0 a 13 días):
Se administrará una primera dosis de carga de 10 mg/kg, para seguir con 7,5 mg/kg cada 12 horas.
Prematuros:
La dosis recomendada es de 7,5 mg/kg cada 12 horas.
Amicacina Braun 500 mg/2ml puede no ser adecuada para ajustar la posología en todos los casos. Existen otras presentaciones de solución para perfusión intravenosa que se puede utilizar en esta situación.
Poblaciones especiales
Insuficiencia renal
En pacientes con insuficiencia renal, medido por un aclaramiento de creatinina < 50 ml/min, no es recomendable la administración de amikacina en dosis única ya que estos pacientes tendrían una exposición prolongada a concentraciones valle elevadas.
La posología puede ajustarse o bien ampliando el intervalo entre las dosis o bien administrando dosis más reducidas.
Ajuste de la posología en función de los valores de creatinina sérica:
- Aumento del intervalo de dosificación, a la dosis normal:
El aumento del intervalo en horas para una dosificación normal (7,5 mg/kg cada 12 horas) se puede calcular multiplicando el valor de creatinina sérica por 9. Por ejemplo, si el valor de creatinina sérica es de 2 mg/100 ml, la posología recomendada sería 7,5 mg/kg cada 18 horas.
- Modificación de la dosis, manteniendo la periodicidad entre administraciones:
Inicialmente se administrará la dosis normal de 7,5 mg/kg, como dosis de carga. Para determinar la dosis de mantenimiento a administrar cada 12 horas, la dosis deberá reducirse en proporción a la reducción del aclaramiento de creatinina:
Dosis de mantenimiento (cada 12 h):
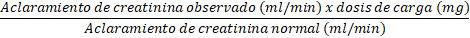
Pacientes con obesidad
La dosis debe ajustarse al peso corporal y a la función renal. En pacientes obesos la dosis inicial debe calcularse con el peso ideal más un 40% de exceso de peso.
Pacientes con quemaduras y con infecciones graves
Pueden necesitar una administración de dosis mayor o a intervalos de cuatro a seis horas debido a que en estos casos la vida media del fármaco es menor.
Forma de administración
Vía intramuscular directa.
Recomendaciones de monitorización:
Para el cálculo de la dosis correcta debe tenerse en cuenta:
- El peso del paciente antes del tratamiento.
- El estado de la función renal determinando la concentración de creatinina sérica o el aclaramiento de creatinina. Debe realizarse un seguimiento de la función renal durante el tratamiento.
Siempre que sea posible, deben determinarse las concentraciones de amikacina en suero, para asegurar niveles adecuados. Se recomienda medir las concentraciones séricas mínimas y máximas intermitentemente durante el tratamiento. Deben evitarse concentraciones máximas (30-90 minutos después de la inyección) superiores a 35 µg/ml y concentraciones mínimas (justo antes de la siguiente dosis) superiores a 10 µg/ml.
Los aminoglucósidos deben ser administrados de forma separada, cualquiera que sea su vía de administración, no debiendo ser físicamente premezclados con otros fármacos.
MEDICAMENTO SUJETO A PRESCRIPCIÓN MÉDICA
USO HOSPITALARIO
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a AMICACINA BRAUN 500 MG/2ML SOLUCIÓN INYECTABLEForma farmacéutica: INYECTABLE PERFUSION, 1000 mgPrincipio activo: amikacinFabricante: B Braun Medical S.A.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 500 mgPrincipio activo: amikacinFabricante: B Braun Medical S.A.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 5 mg/mlPrincipio activo: amikacinFabricante: Fresenius Kabi España, S.A.U.Requiere receta
Médicos online para AMICACINA BRAUN 500 MG/2ML SOLUCIÓN INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de AMICACINA BRAUN 500 MG/2ML SOLUCIÓN INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















