
ACULAR 5 mg/ml COLIRIO EN SOLUCION

Cómo usar ACULAR 5 mg/ml COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
ACULAR 5 mg/ml colirio en solución
(ketorolaco trometamol)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es ACULAR y para qué se utiliza
- Qué necesita saber antes de empezar a usar ACULAR
- Cómo usar ACULAR
- Posibles efectos adversos
5 Conservación de ACULAR
- Contenido del envase e información adicional
1. Qué es ACULAR y para qué se utiliza
ACULAR se utiliza para prevenir y reducir la inflamación ocular tras la cirugía de cataratas en adultos.
ACULAR pertenece a un grupo de medicamentos conocidos como antiinflamatorios no esteroideos (AINES).
2. Qué necesita saber antes de empezar a usar ACULAR
No use ACULAR
- Si es alérgico al ketorolaco trometamol, o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- · Si es alérgico a la aspirina o a otros medicamentos similares, tales como otros medicamentos antiinflamatorios no esteroideos.
Advertencias y precauciones
Consulte a su médico,farmacéutico o enfermero antes de empezar a usar ACULAR.
Si usted tiene o ha tenido en el pasado:
- Infecciones oculares bacterianas o virales
- Tendencia a sufrir hemorragias (por ejemplo: anemia) o úlceras de estómago
- Diabetes
- Artritis reumatoide
- Síndrome del ojo seco
- Asma después de utilizar antiinflamatorios no esteroideos
- Si ha tenido una operación del ojo reciente.
- Si ha perdido sensibilidad en la córnea ( la capa transparente que cubre la pupila y el iris) o si la superficie normalmente lisa de la córnea está dañada.
Niños y adolescentes
ACULAR no se debe prescribir para su uso en niños.
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Si utiliza ACULAR con cualquier otro medicamento por vía oftálmica, deje pasar al menos 5 minutos entre la administración de ACULAR y el otro medicamento.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento
ACULAR no debe utilizarse si está embarazada o está en período de lactancia, a menos que el médico lo recomiende.
Conducción y uso de máquinas
ACULAR puede causar visión borrosa en algunos pacientes. No conduzca ni use máquinas hasta que los síntomas hayan desaparecido.
ACULAR contiene cloruro de benzalconio
Este medicamento contiene 0,1 mg de cloruro de benzalconio en cada mililitro equivalente a 0,1 mg/ml.
El cloruro de benzalconio se puede absorber por las lentes de contacto blandas alterando su color. Retirar las lentes de contacto antes de usar este medicamento y esperar 15 minutos antes de volver a colocarlas.
El cloruro de benzalconio puede causar irritación ocular, especialmente si padece de ojo seco u otras enfermedades de la córnea (capa transparente de la zona frontal del ojo). Consulte a su médico si siente una sensación extraña, escozor o dolor en el ojo después de usar este medicamento.
3. Cómo usar ACULAR
Siga exactamente las instrucciones de administración de ACULAR indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico. La dosis recomendada es una gota en el ojo afectado 3 veces al día durante 3-4 semanas después de la cirugía de cataratas, comenzando 24 horas antes de la operación.
Instrucciones de uso
Aplíquese el colirio de la siguiente manera:
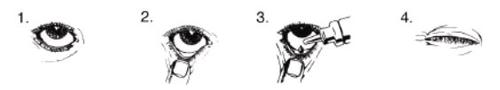
- Lávese las manos. Incline la cabeza hacia atrás y mire hacia el techo.
- Tire suavemente hacia abajo del párpado inferior, hasta que haga un pequeño hueco.
- Gire el envase hacia abajo y apriételo para que caigan una gota en cada ojo que necesite tratamiento.
- Suelte el párpado inferior y mantenga el ojo cerrado durante 30 segundos.
Si una gota cae fuera del ojo repita la operación.
Para evitar la contaminación o una lesión, no permita que la punta del envase toque el ojo u otra
superficie.
Vuelva a poner el tapón y cierre el envase inmediatamente después de usarlo.
Limpie cualquier exceso de líquido de su mejilla con un pañuelo limpio.
Es muy importante una aplicación correcta de su colirio.
Si tiene cualquier duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Si usa más ACULAR del que debe
La aplicación de un exceso de gotas es poco probable que produzca efectos adversos indeseados. Aplique su siguiente dosis a la hora normal. Si por accidente, alguien ingiere este medicamento, beba líquidos para diluir y consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20.
Se recomienda llevar el prospecto y el envase del medicamento al profesional sanitario.
Si olvidó usar ACULAR
Si olvidó usar ACULAR, aplíquelo tan pronto como se acuerde, a menos que sea casi la hora de la siguiente dosis, en cuyo caso sáltese la dosis olvidada. Aplique su siguiente dosis y continúe con su pauta habitual. No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con ACULAR
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas).
- Irritación del ojo
- picor y /o quemazón en el ojo
- dolor ocular.
Frecuentes(pueden afectar a hasta 1 de cada 10 personas)
- Reacción alérgica
- hinchazón/inflamación del ojo o del párpado
- picor de ojos
- ojo enrojecido
- infección del ojo
- inflamación del ojo (superficie o interior)
- formación de pequeños depósitos en la capa transparente en la parte frontal del ojo
- sangrado de la retina
- inflamación de la retina central (capa fotosensible del ojo)
- dolor de cabeza
- lesión causada accidentalmente por el contacto del gotero y el ojo
- presión aumentada en el ojo
- visión borrosa y/o disminuida.
Poco frecuentes(pueden afectar a hasta 1 de cada 100 personas)
- Inflamación o daño en la capa transparente en la parte delantera del ojo
- sequedad ocular y/o ojos llorosos.
Frecuencia no conocida(la frecuencia no puede estimarse a partir de los datos disponibles)
- Daño en la superficie del ojo, tal como adelgazamiento
- erosión
- perforación
- degradación de la(s) célula(s)
- dificultad para respirar o sibilancias
- empeoramiento del asma
- inflamación de la cara
- úlcera en la superficie del ojo.
Los efectos adversos relacionadas con la córnea (la superficie del ojo) pueden ser más probables si se usa ACULAR durante más de dos semanas o si está usando colirios corticosteroides al mismo tiempo o si tiene una patología ocular relacionada. Usted debe consultar a su médico si experimenta dolor, un aumento de la irritación en el ojo y cambios en la visión.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ACULAR
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del envase y en la caja después de EXP (corresponde a las siglas en inglés de caducidad). La fecha de caducidad es el último día del mes que se indica.
Deseche el medicamento 28 días después de abierto, aunque todavía quede algo de solución.
No conservar a temperatura superior a 25 º C.
No utilice este medicamento si observa que el sello de protección está roto.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ACULAR
- El principio activo es Ketorolaco trometamol 5 mg/ml.
- Los demás componentes son cloruro de benzalconio, edetato disódico, octoxinol 40, cloruro sódico, hidróxido sódico o ácido clorhídrico (para ajustar el pH) y agua purificada (ver sección 2).
Aspecto del producto y contenido del envase
ACULAR es un colirio en solución transparente de incoloro a ligeramente amarillento, en un envase de plástico.
Cada envase contiene 1 frasco de plástico de 10 ml de capacidad provisto de tapón de rosca.
Cada envase contiene 5 ó 10 mililitros de solución.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
AbbVie Spain, S.L.U.
Avenida de Burgos 91
28050, Madrid
España
Responsable de la fabricación:
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport
County Mayo
Irlanda
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria | ACULAR 0.5% Augentropfen |
Bélgica | ACULARE oogdruppels |
Dinamarca, Irlanda, Italia, Reino Unido | ACULAR |
Portugal | ACULAR 0,5% p/v, colírio solução |
España | ACULAR 5 mg/ml colirio en solución |
Finlandia | ACULAR 5 mg/ml eye drops |
Francia | ACULAR 0,5% |
Grecia | ACULAR 0,5% |
Luxemburgo | ACULARE collyre |
Holanda | ACULAR oogdruppels 0,5% |
Alemania | ACULAR 5 mg/ml Augentropfen |
Fecha de la última revisión de este prospecto:04/2025
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
Para solicitar una copia de este prospecto en tamaño de letra grande, póngase en contacto con el representante local del titular de la autorización de comercialización.
/
- País de registro
- Precio medio en farmacia5.18 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ACULAR 5 mg/ml COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 1 mg/mlPrincipio activo: DiclofenacoFabricante: Laboratoires TheaRequiere recetaForma farmacéutica: COLIRIO, 1 MG/MLPrincipio activo: DiclofenacoFabricante: Qualix Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 0,1% diclofenaco sodicoPrincipio activo: DiclofenacoFabricante: Angelini Pharma Espana S.L.Requiere receta
Médicos online para ACULAR 5 mg/ml COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ACULAR 5 mg/ml COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















