ACTILYSE POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE Y PARA PERFUSION

Cómo usar ACTILYSE POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE Y PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Actilyse, polvo y disolvente para solución inyectable y para perfusión
alteplasa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Actilyse y para qué se utiliza
- Qué necesita saber antes de recibir Actilyse
- Cómo se administra Actilyse
- Posibles efectos adversos
- Conservación de Actilyse
- Contenido del envase e información adicional
1. Qué es Actilyse y para qué se utiliza
El principio activo de Actilyse es la alteplasa. Pertenece a un grupo de medicamentos denominados trombolíticos. Estos medicamentos actúan disolviendo los coágulos de sangre que se han formado en los vasos sanguíneos.
Actilyse 10 mg, 20 mg o 50 mg se utiliza para tratar enfermedades causadas por la formación de coágulos de sangre dentro de los vasos sanguíneos, incluyendo:
- ataques al corazón causados por coágulos de sangre en las arterias del corazón (infarto agudo de miocardio)
- coágulos de sangre en las arterias de los pulmones (embolia pulmonar aguda masiva)
- ictus causado por un coágulo de sangre en una arteria del cerebro (ictus isquémico agudo)
2. Qué necesita saber antes de recibir Actilyse
Usted no debe recibir Actilyse:
- si es alérgico (hipersensible) a alteplasa o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene o ha tenido recientemente una enfermedad que aumenta el riesgo de hemorragia, incluyendo:
- trastorno hemorrágico o predisposición al sangrado
- hemorragia grave o peligrosa en cualquier parte del cuerpo
- hemorragia dentro del cerebro o del cráneo
- tensión arterial muy elevada no controlada
- infección bacteriana o inflamación del corazón (endocarditis), o inflamación de las membranas que envuelven el corazón (pericarditis)
- inflamación del páncreas (pancreatitis aguda)
- úlcera gástrica o úlceras en el intestino
- venas varicosas en el esófago (varices esofágicas)
- anomalías de los vasos sanguíneos, como por ejemplo el ensanchamiento localizado de una arteria (aneurisma)
- determinados tumores
- enfermedad grave del hígado
- si está tomando medicamentos para “diluir” la sangre (anticoagulantes orales), a menos que las pruebas apropiadas no muestren actividad clínicamente relevante de dicho medicamento
- si ha sido sometido alguna vez a cirugía en el cerebro o en la médula espinal
- si ha sido sometido a cirugía mayor o a un traumatismo importante durante los últimos 3 meses
- si ha recibido una punción reciente en un vaso sanguíneo mayor
- si se le ha practicado un masaje cardíaco externo durante los últimos 10 días
- si ha tenido un bebé en los últimos 10 días
Su médico tampoco le administrará Actilyse para el tratamiento de ataques al corazón o coágulos de sangre en las arterias de los pulmones
- si tiene o ha tenido alguna vez un ictus producido por hemorragia en el cerebro (ictus hemorrágico)
- si tiene o ha tenido alguna vez un ictus de causa desconocida
- si ha tenido recientemente (en los últimos 6 meses) un ictus causado por un coágulo de sangre en una arteria del cerebro (ictus isquémico), a menos que sea el ictus por el que le van a tratar ahora
Además su médico no le administrará Actilyse para el tratamiento del ictus causado por un coágulo de sangre en una arteria del cerebro (ictus isquémico agudo)
- si los síntomas de ictus empezaron hace más de 4,5 horas o si es posible que los síntomas empezaran hace más de 4,5 horas porque desconoce cuándo empezaron
- si su ictus presenta solamente síntomas muy leves
- si hay signos de hemorragia en el cerebro
- si ha tenido un ictus en los últimos tres meses
- si los síntomas mejoran rápidamente antes de que le sea administrado Actilyse
- si presenta un ictus muy grave
- si tenía espasmos (convulsiones) cuando se inició el ictus
- si presenta un tiempo de tromboplastina (un test para comprobar cómo coagula su sangre) anormal. Este test puede ser anormal si se le ha administrado heparina (un medicamento utilizado para “diluir” la sangre) durante las últimas 48 horas
- si es diabético y ha sufrido un ictus alguna vez
- si la cantidad de plaquetas (trombocitos) en su sangre es muy baja
- si su tensión arterial es muy elevada (por encima de 185/110) y solo puede reducirse cuando se le administran medicamentos
- si los niveles de azúcar (glucosa) en sangre son muy bajos (menos de 50 mg/dl)
- si los niveles de azúcar (glucosa) en sangre son muy altos (más de 400 mg/dl)
- si tiene menos de 16 años. (Para adolescentes de 16 años o mayores, ver sección “Su médico tendrá especial cuidado con Actilyse”).
Su médico tendrá especial cuidado con Actilyse
- si ha sufrido cualquier reacción alérgica diferente a una reacción alérgica repentina y potencialmente mortal (hipersensibilidad grave) al principio activo alteplasa o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene o ha tenido recientemente cualquier otro trastorno que aumente el riesgo de hemorragia, tal y como:
- traumatismo menor
- biopsia (un procedimiento utilizado para obtener muestras de tejidos)
- punción de vasos mayores
- inyección intramuscular
- masaje cardíaco externo
- si se le ha administrado Actilyse alguna vez
- si tiene más de 65 años de edad
- si tiene más de 80 años de edad, puede tener un peor resultado independientemente del tratamiento con Actilyse. Sin embargo, en general el beneficio-riesgo con Actilyse en pacientes de más de 80 años es positivo y la edad por sí sola no constituye una barrera para el tratamiento con Actilyse
- si es un adolescente de 16 años o mayor se debe evaluar cuidadosamente la relación beneficio-riesgo de forma individual para el tratamiento de ictus isquémico agudo
Otros medicamentos y Actilyse
Informe a su médico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta. Es especialmente importante que informe a su médico si está utilizando o ha utilizado recientemente:
- medicamentos utilizados para “diluir” la sangre, incluyendo:
- ácido acetilsalicílico
- warfarina
- cumarina
- heparina
- algunos medicamentos utilizados para tratar la tensión arterial alta (inhibidores del Enzima Convertidor de Angiotensina, ECA)
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico. Su médico le administrará Actilyse solamente si los beneficios esperados superan el riesgo para su bebé.
3. Cómo se administra Actilyse
Actilyse lo preparará y le será administrado por su médico o un profesional de la salud. No está indicado para ser auto-administrado.
El tratamiento con Actilyse debe iniciarse lo antes posible después de la presentación de los síntomas.
Hay tres enfermedades para las cuales Actilyse está indicado:
Ataque al corazón (infarto agudo de miocardio)
La dosis que le será administrada dependerá de su peso corporal. La dosis máxima de Actilyse es 100 mg pero será inferior si pesa menos de 65 kg.
Puede administrarse de dos maneras distintas:
- Régimen de dosificación de 90 minutos, para pacientes tratados durante las 6 horas posteriores a la presentación de los síntomas. Consiste en:
- una inyección inicial de parte de la dosis de Actilyse en una vena
- perfusión del resto de la dosis durante los 90 minutos siguientes.
- Régimen de dosificación de 3 horas, para pacientes tratados durante las 6‑12 horas posteriores a la presentación de los síntomas. Consiste en:
- una inyección inicial de parte de la dosis de Actilyse en una vena
- perfusión del resto de la dosis durante las 3 horas siguientes.
Además de Actilyse su médico le administrará otro medicamento para impedir la formación de coágulos. Este medicamento le será administrado tan pronto como sea posible después del inicio del dolor en el pecho.
Coágulos de sangre en las arterias de los pulmones (embolia pulmonar aguda masiva)
La dosis que le será administrada dependerá de su peso corporal. La dosis máxima de Actilyse es 100 mg pero será inferior si pesa menos de 65 kg.
Normalmente el medicamento se administra así:
- una inyección inicial de parte de la dosis en una vena
- perfusión del resto de la dosis durante las 2 horas siguientes.
Después del tratamiento con Actilyse, su médico comenzará (o continuará) el tratamiento con heparina (un medicamento utilizado para “diluir” la sangre).
Ictus causado por un coágulo de sangre en una arteria del cerebro (ictus isquémico agudo)
Actilyse debe administrarse durante las 4,5 horas posteriores a la presentación de los primeros síntomas. Cuanto antes reciba Actilyse, más se puede beneficiar del tratamiento y menor es la probabilidad de que aparezcan efectos adversos perjudiciales. La dosis que le será administrada dependerá de su peso corporal. La dosis máxima de este medicamento es 90 mg pero será inferior si pesa menos de 100 kg. Actilyse se administra así:
- una inyección inicial de parte de la dosis en una vena
- perfusión del resto de la dosis durante los 60 minutos siguientes.
No debe tomar ácido acetilsalicílico durante las primeras 24 horas después de ser tratado de un ictus con Actilyse. Su médico podría administrarle una inyección de heparina si fuera necesario.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o a un profesional de la salud.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos descritos a continuación han sido observados en pacientes a los que se administró Actilyse:
Muy frecuentes (pueden afectar a más de 1 de cada 10 pacientes)
- fallo cardíaco - puede ser necesaria la interrupción del tratamiento
- hemorragia en el cerebro (hemorragia cerebral) tras el tratamiento de un ictus causado por un coágulo de sangre en una arteria del cerebro (ictus isquémico agudo)- puede ser necesaria la interrupción del tratamiento
- líquido en los pulmones (edema pulmonar)
- sangrado en el vaso sanguíneo dañado (como hematoma)
- tensión arterial baja (hipotensión)
- dolor en el pecho (angina de pecho)
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- más ataques al corazón
- hemorragia en el cerebro (hemorragia cerebral) tras el tratamiento de un ataque al corazón (infarto de miocardio) - puede ser necesaria la interrupción del tratamiento
- cese de los latidos del corazón (paro cardíaco) - puede ser necesaria la interrupción del tratamiento
- shock (tensión arterial muy baja) debido a fallo cardíaco - puede ser necesaria la interrupción del tratamiento
- hemorragia en la garganta
- hemorragia en el estómago o intestino, incluyendo sangre en vómitos (hematemesis) o sangre en heces (melenas o hemorragia rectal), hemorragia en las encías
- hemorragia en tejidos corporales causando cardenales purpúreos (equimosis)
- hemorragia del tracto urinario o de los órganos reproductores, que puede originar la presencia de sangre en la orina (hematuria)
- hemorragia o formación de cardenales (hematoma) en el lugar de inyección
Poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes)
- hemorragia relacionada con el pulmón, como flema manchada con sangre (hemoptisis) o hemorragia en el tracto respiratorio - puede ser necesaria la interrupción del tratamiento
- hemorragias nasales (epistaxis)
- latidos irregulares del corazón después de que el aporte de sangre al corazón se haya restaurado
- lesiones en las válvulas del corazón (regurgitación mitral) o en las paredes que dividen las cavidades del corazón (defecto del tabique ventricular) - puede ser necesaria la interrupción del tratamiento
- bloqueo repentino de una arteria de los pulmones (embolia pulmonar), del cerebro (embolia cerebral) y de otras áreas del cuerpo (embolia sistémica)
- hemorragia de la oreja
- disminución de la presión sanguínea
Raros (pueden afectar hasta 1 de cada 1.000 pacientes)
- hemorragia en la capa membranosa que envuelve el corazón (hemopericardio) - puede ser necesaria la interrupción del tratamiento
- hemorragia interna en la parte posterior del abdomen (hemorragia retroperitoneal) - puede ser necesaria la interrupción del tratamiento
- formación de coágulos de sangre en vasos sanguíneos que pueden llegar a otros órganos del cuerpo (embolismo). Los síntomas dependerán del órgano afectado
- reacciones alérgicas, por ejemplo, ronchas (urticaria) y erupción, dificultad para respirar hasta asma (broncoespasmo), líquido debajo de la piel y de la membrana mucosa (angioedema), tensión arterial baja o shock – puede ser necesaria la interrupción del tratamiento
- hemorragia en el ojo (hemorragia ocular)
- indisposición del estómago (náuseas)
Muy raros (pueden afectar hasta 1 de cada 10.000 pacientes)
- reacciones alérgicas graves (p. ej. anafilaxia que suponga una amenaza para la vida) - puede ser necesaria la interrupción del tratamiento
- acontecimientos que afectan el sistema nervioso como por ejemplo:
- espasmos (convulsiones, ataques)
- dificultad para hablar
- confusión o delirio (confusión muy grave)
- ansiedad acompañada de inquietud (agitación)
- depresión
- pensamientos alterados (psicosis)
Estos trastornos suelen suceder a menudo asociados a un ictus causado por un coágulo de sangre o hemorragia en el cerebro.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- hemorragia en órganos internos, por ejemplo hemorragia en el hígado (hemorragia hepática) - puede ser necesaria la interrupción del tratamiento
- formación de cristales de colesterol, que pueden llegar a otros órganos del cuerpo (embolización por cristales de colesterol). Los síntomas dependerán del órgano afectado - puede ser necesaria la interrupción del tratamiento
- hemorragia que pueda requerir una transfusión sanguínea
- vómitos
- aumento de la temperatura del cuerpo (fiebre)
Los pacientes que han padecido una hemorragia en el cerebro u otros acontecimientos de hemorragias graves pueden morir o sufrir discapacidad permanente.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Actilyse
No es habitual que usted deba conservar Actilyse ya que le será administrado por su médico.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25 ºC. Conservar en el embalaje original para protegerlo de la luz.
No utilice Actilyse después de la fecha de caducidad que aparece en la etiqueta del vial y en el envase. La fecha de caducidad es el último día del mes que se indica.
Solución reconstituida
La solución reconstituida ha demostrado ser estable durante 24 horas a 2 ºC‑8 ºC y durante 8 horas a 25 ºC.
Desde un punto de vista microbiológico, el producto debe ser usado inmediatamente tras su reconstitución. Si no se usa inmediatamente, el período de conservación y las condiciones de uso antes de su utilización serán responsabilidad de la persona que lo utilice y no debería ser superior a 24 horas a 2‑8 ºC.
6. Contenido del envase e información adicional
Composición de Actilyse
- El principio activo es alteplasa. Cada vial contiene 10 mg (equivalente a 5.800.000 UI), 20 mg (equivalente a 11.600.000 UI), ó 50 mg (equivalente a 29.000.000 UI) de alteplasa.
La alteplasa es producida mediante la técnica de ADN recombinante, utilizando una línea celular ovárica de hámster chino. Los demás componentes son arginina, ácido fosfórico (para el ajuste del pH) y polisorbato 80.
- El disolvente es agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Actilyse es polvo y disolvente para solución inyectable y para perfusión. Cada envase contiene un vial con polvo y un vial con disolvente.
Actilyse está disponible en los siguientes tamaños de envases:
- un vial de polvo con 10 mg de alteplasa y un vial con 10 ml de disolvente.
- un vial de polvo con 20 mg de alteplasa, un vial con 20 ml de disolvente y una cánula de transferencia.
- un vial de polvo con 50 mg de alteplasa, un vial con 50 ml de disolvente y una cánula de transferencia.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Boehringer Ingelheim International GmbH
55216 Ingelheim am Rhein
Alemania
Representante local:
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
España
Responsable de la fabricación:
Boehringer Ingelheim Pharma GmbH & Co. KG
Birkendorfer Strasse 65
88397 Biberach/Riss
Alemania
Boehringer Ingelheim France
100-104 avenue de France
75013 Paris
Francia
Fecha de la última revisión de este prospecto:09/2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
-------------------------------------------------------------------------------------------------------------------------------
La siguiente información está destinada solo a profesionales sanitarios:
Trazabilidad
Con objeto de mejorar la trazabilidad de los medicamentos biológicos, el nombre y el número de lote del medicamento administrado deben estar claramente registrados.
Los viales de 2 mg de alteplasa no están indicados para utilizarse en las indicaciones de infarto agudo de miocardio, embolia pulmonar aguda masiva o ictus isquémico agudo (debido al riesgo de infradosificación masiva). Solamente los viales de 10 mg, 20 mg y 50 mg están indicados para utilizarse en estas indicaciones.
Reconstitución
Para obtener una concentración final de 1 mg de alteplasa por ml tras la reconstitución, se debe transferir todo el volumen del disolvente proporcionado al vial que contiene el polvo de Actilyse. Con este propósito debe utilizarse la cánula de transferencia que se incluye en los tamaños de envases de 20 mg y 50 mg. Para la presentación de 10 mg debe utilizarse una jeringa.
Para obtener una concentración final de 2 mg de alteplasa por ml tras la reconstitución, solo debe utilizarse la mitad del disolvente proporcionado (según la tabla de abajo). En estos casos, siempre debe utilizarse una jeringa para transferir la cantidad necesaria de disolvente al vial que contiene el polvo de Actilyse.
El contenido de un vial para inyección de Actilyse (10 mg, 20 mg ó 50 mg) debe disolverse bajo condiciones asépticas con agua para preparaciones inyectables, según la tabla siguiente, para obtener una concentración final de alteplasa de 1 mg/ml o de 2 mg/ml:
Actilyse sustancia seca | 10 mg | 20 mg | 50 mg |
(a) Volumen de agua esterilizada para preparaciones inyectables que se debe añadir a la sustancia seca | 10 ml | 20 ml | 50 ml |
Concentración final: | 1 mg alteplasa/ml | 1 mg alteplasa/ml | 1 mg alteplasa/ml |
(b) Volumen de agua esterilizada para preparaciones inyectables que se debe añadir a la sustancia seca | 5 ml | 10 ml | 25 ml |
Concentración final: | 2 mg alteplasa/ml | 2 mg alteplasa/ml | 2 mg alteplasa/ml |
La solución reconstituida debe administrarse a continuación por vía intravenosa. La solución reconstituida de 1 mg/ml puede diluirse adicionalmente con una solución inyectable estéril de cloruro de sodio 9 mg/ml (0,9 %) hasta una concentración mínima de 0,2 mg/ml ya que no se puede excluir la aparición de turbidez de la solución reconstituida. No se recomienda la dilución adicional de la solución reconstituida de 1 mg/ml con agua esterilizada para preparaciones inyectables o, en general, el uso de soluciones de carbohidratos para perfusión, p. ej. dextrosa, debido al aumento de formación de turbidez en la solución reconstituida. Actilyse no debe mezclarse con otros medicamentos en el mismo vial de perfusión (ni siquiera con heparina).
Para condiciones de conservación, por favor ver sección 5 del prospecto.
La solución reconstituida es de administración única. Cualquier fracción de solución no utilizada debe desecharse.
Instrucciones para reconstituir Actilyse
1 | Reconstituir inmediatamente antes de su administración. |
|
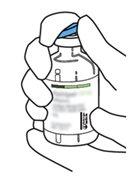
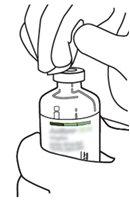
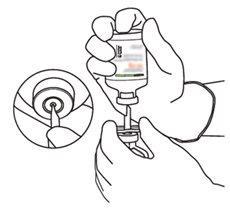
2 | Retirar las tapas protectoras de los dos viales que contienen agua estéril y Actilyse sustancia seca, respectivamente, tirándolas hacia arriba con un dedo. |
|
3 | Limpiar el tapón de goma de cada uno de los viales con una toallita con alcohol. |
|
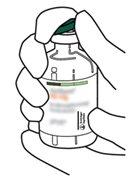
4 | Sacar la cánula de transferencia* de su envoltorio. No desinfectar ni esterilizar la cánula de transferencia; es estéril. Quitar la tapa. |
|
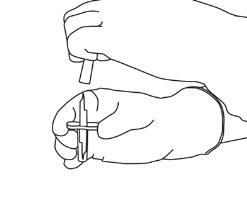
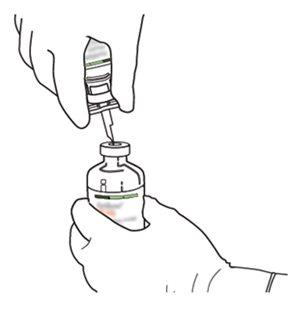
5 | Mantener el vial de agua estéril vertical sobre una superficie estable. Directamente desde arriba, perforar el tapón de goma verticalmente en el centro del tapón con la cánula de transferencia, presionando con cuidado pero firmemente, sin girar. |
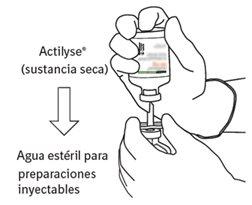
|
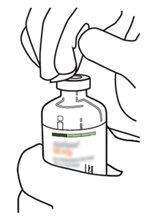
6 | Sujetar el vial de agua estéril y la cánula de transferencia firmemente con una mano utilizando las dos solapas laterales. Retirar la tapa restante de la parte superior de la cánula de transferencia. |
|
7 | Sujetar el vial de agua estéril y la cánula de transferencia firmemente con una mano utilizando las dos solapas laterales. Sujetar el vial con Actilyse sustancia seca verticalmente encima de la cánula de transferencia y posicionar la punta de la cánula de transferencia justo en el centro del tapón. Presionar el vial con la sustancia seca hacia abajo con la cánula de transferencia directamente desde arriba, perforando el tapón de goma verticalmente y con cuidado pero firmemente, sin girar. |
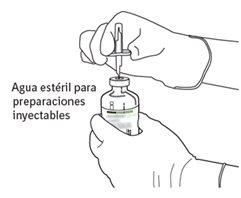
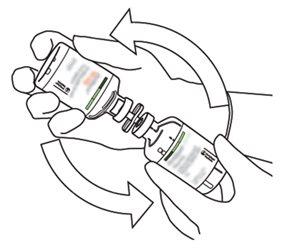
|
8 | Invertir los dos viales y permitir que el agua drene completamente en la sustancia seca. |
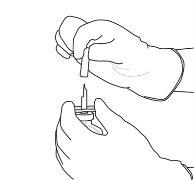
|
9 | Retirar el vial de agua vacío junto con la cánula de transferencia. Se pueden desechar. |
|
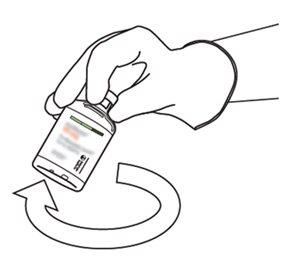
10 | Coger el vial con Actilyse reconstituido y girarlo con cuidado para disolver cualquier polvo restante pero no agitar, puesto que esto producirá espuma. Si hay burbujas, mantener la solución inmóvil durante unos minutos para permitir que desaparezcan. |
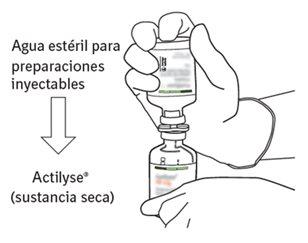
|
11 | La solución reconstituida contiene 1 mg/ml de alteplasa. Debe ser límpida y de incolora a amarilla clara y no debe contener ninguna partícula. | |
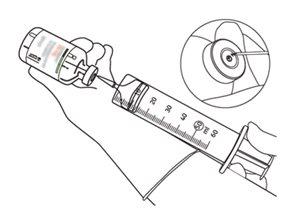
12 | Extraer la cantidad requerida solamente utilizando una aguja y una jeringa. No utilizar la zona de punción de la cánula de transferencia para evitar pérdidas. |
|
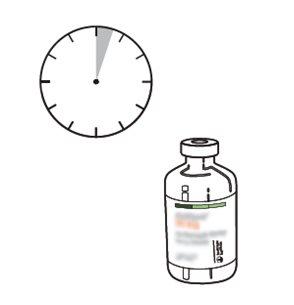
13 | Utilizar inmediatamente. Desechar la solución no utilizada. |
(* Si se incluye en el kit una cánula de transferencia. La reconstitución también se puede realizar con una jeringa y una aguja.)
Posología y forma de administración
Infarto agudo de miocardio
Posología
- Régimen de dosificación de 90 minutos (acelerado) para pacientes con infarto agudo de miocardio, en los cuales pueda iniciarse el tratamiento dentro de las 6 horas después de la presentación de los síntomas.
En pacientes con un peso corporal ≥ 65 kg:
Volumen a administrar en función de la concentración de alteplasa | ||
1 mg/ml | 2 mg/ml | |
15 mg en forma de bolo intravenoso, inmediatamente seguido de | 15 ml | 7,5 ml |
50 mg en forma de perfusión intravenosa a velocidad constante durante los primeros 30 minutos, inmediatamente seguido de | 50 ml | 25 ml |
35 mg en forma de perfusión intravenosa a velocidad constante durante 60 minutos, hasta una dosis máxima total de 100 mg | 35 ml | 17,5 ml |
En pacientes con un peso corporal < 65 kg, la dosis total debe ajustarse al peso de la siguiente forma:
Volumen a administrar en función de la concentración de alteplasa | ||
1 mg/ml | 2 mg/ml | |
15 mg en forma de bolo intravenoso, inmediatamente seguido de | 15 ml | 7,5 ml |
0,75 mg/kg de peso corporal (p.c.) en forma de perfusión intravenosa a velocidad constante durante los primeros 30 minutos, inmediatamente seguido de | 0,75 ml/kg p.c. | 0,375 ml/kg p.c. |
0,5 mg/kg de peso corporal (p.c.) en forma de perfusión intravenosa a velocidad constante durante 60 minutos | 0,5 ml/kg p.c. | 0,25 ml/kg p.c. |
- Régimen de dosificación de 3 horas para pacientes con infarto agudo de miocardio en los cuales pueda iniciarse el tratamiento entre las 6 y 12 horas después de la presentación de los síntomas.
En pacientes con un peso corporal ≥ 65 kg:
Volumen a administrar en función de la concentración de alteplasa | ||
1 mg/ml | 2 mg/ml | |
10 mg en forma de bolo intravenoso, inmediatamente seguido de | 10 ml | 5 ml |
50 mg en forma de perfusión intravenosa a velocidad constante durante la primera hora, inmediatamente seguido de | 50 ml | 25 ml |
40 mg en forma de perfusión intravenosa a velocidad constante durante 2 horas, hasta una dosis máxima total de 100 mg | 40 ml | 20 ml |
En pacientes con un peso corporal < 65 kg:
Volumen a administrar en función de la concentración de alteplasa | ||
1 mg/ml | 2 mg/ml | |
10 mg en forma de bolo intravenoso, inmediatamente seguido de | 10 ml | 5 ml |
una perfusión intravenosa a velocidad constante durante 3 horas hasta una dosis máxima total de 1,5 mg/kg p.c. | 1,5 ml/kg p.c. | 0,75 ml/kg p.c. |
Tratamiento coadyuvante: Se recomienda tratamiento antitrombótico coadyuvante en cumplimiento con las guías internacionales actuales para el tratamiento de pacientes con infarto de miocardio con elevación del ST.
Forma de administración
La solución reconstituida debe administrarse por vía intravenosa y es para uso inmediato.
Los viales de 2 mg de alteplasa no están indicados para ser usados en esta indicación.
Embolia pulmonar aguda masiva
Posología
En pacientes con un peso corporal ≥ 65 kg:
Debe administrarse una dosis total de 100 mg de alteplasa en 2 horas. El siguiente régimen de dosificación es con el que se tiene mayor experiencia:
Volumen a administrar en función de la concentración de alteplasa | ||
1 mg/ml | 2 mg/ml | |
10 mg en forma de bolo intravenoso durante 1-2 minutos, inmediatamente seguido de | 10 ml | 5 ml |
90 mg como perfusión intravenosa a velocidad constante durante 2 horas hasta una dosis máxima total de 100 mg | 90 ml | 45 ml |
En pacientes con un peso corporal < 65 kg:
Volumen a administrar en función de la concentración de alteplasa | ||
1 mg/ml | 2 mg/ml | |
10 mg en forma de bolo intravenoso durante 1-2 minutos, inmediatamente seguido de | 10 ml | 5 ml |
una perfusión intravenosa a velocidad constante durante 2 horas hasta una dosis máxima total de 1,5 mg/kg p.c. | 1,5 ml/kg p.c. | 0,75 ml/kg p.c. |
Tratamiento coadyuvante: Después del tratamiento con Actilyse debe iniciarse (o reanudarse) un tratamiento con heparina si los valores aPTT son inferiores al doble del límite superior normal. La perfusión debe ajustarse para mantener los valores de aPTT en el rango de 50 – 70 segundos (de 1,5 a 2,5 veces el valor de referencia).
Forma de administración
La solución reconstituida debe administrarse por vía intravenosa y es para uso inmediato.
Los viales de 2 mg de alteplasa no están indicados para ser usados en esta indicación.
Ictus isquémico agudo
El tratamiento solo debe ser realizado bajo la responsabilidad y supervisión de un médico entrenado y con experiencia en cuidados neurovasculares, ver Ficha técnica sección 4.3 contraindicaciones y 4.4 advertencias y precauciones especiales de empleo.
El tratamiento con Actilyse debe iniciarse tan pronto como sea posible dentro de las 4,5 horas desde la presentación de los síntomas (ver Ficha técnica sección 4.4). Más allá de las 4,5 horas después de la presentación de los síntomas de ictus hay una relación beneficio-riesgo negativa asociada al tratamiento con Actilyse y no se debe administrar (ver Ficha técnica sección 5.1).
Posología
La dosis total recomendada es de 0,9 mg de alteplasa/kg de peso corporal (hasta un máximo de 90 mg) empezando con un 10% de la dosis total en forma de bolo intravenoso inicial, inmediatamente seguido del resto de la dosis total perfundida por vía intravenosa durante 60 minutos.
TABLA DE DOSIFICACIÓN PARA ICTUS ISQUÉMICO AGUDO | |||
Utilizando la concentración estándar recomendada de 1 mg/ml el volumen (ml) a administrar es igual al valor de dosis recomendado (mg) | |||
Peso (kg) | Dosis Total (mg) | Dosis Bolo (mg) | Dosis Perfusión* (mg) |
40 | 36,0 | 3,6 | 32,4 |
42 | 37,8 | 3,8 | 34,0 |
44 | 39,6 | 4,0 | 35,6 |
46 | 41,4 | 4,1 | 37,3 |
48 | 43,2 | 4,3 | 38,9 |
50 | 45,0 | 4,5 | 40,5 |
52 | 46,8 | 4,7 | 42,1 |
54 | 48,6 | 4,9 | 43,7 |
56 | 50,4 | 5,0 | 45,4 |
58 | 52,2 | 5,2 | 47,0 |
60 | 54,0 | 5,4 | 48,6 |
62 | 55,8 | 5,6 | 50,2 |
64 | 57,6 | 5,8 | 51,8 |
66 | 59,4 | 5,9 | 53,5 |
68 | 61,2 | 6,1 | 55,1 |
70 | 63,0 | 6,3 | 56,7 |
72 | 64,8 | 6,5 | 58,3 |
74 | 66,6 | 6,7 | 59,9 |
76 | 68,4 | 6,8 | 61,6 |
78 | 70,2 | 7,0 | 63,2 |
80 | 72,0 | 7,2 | 64,8 |
82 | 73,8 | 7,4 | 66,4 |
84 | 75,6 | 7,6 | 68,0 |
86 | 77,4 | 7,7 | 69,7 |
88 | 79,2 | 7,9 | 71,3 |
90 | 81,0 | 8,1 | 72,9 |
92 | 82,8 | 8,3 | 74,5 |
94 | 84,6 | 8,5 | 76,1 |
96 | 86,4 | 8,6 | 77,8 |
98 | 88,2 | 8,8 | 79,4 |
100+ | 90,0 | 9,0 | 81,0 |
*administrado en una concentración de 1mg/ml durante 60 min a una velocidad de perfusión constante.
Tratamiento coadyuvante:la seguridad y eficacia de este régimen con la administración concomitante de heparina o inhibidores de la agregación plaquetaria como el ácido acetilsalicílico durante las primeras 24 horas después de la presentación de los síntomas no han sido suficientemente investigadas. Por lo tanto, la administración de heparina intravenosa o inhibidores de la agregación plaquetaria como el ácido acetilsalicílico debe evitarse en las primeras 24 horas después del tratamiento con Actilyse debido a un incremento del riesgo de hemorragia. Si se requiere heparina para otras indicaciones (p.ej. prevención de la trombosis venosa profunda) la dosis no debe exceder las 10.000 UI por día, administrada por vía subcutánea.
Forma de administración
La solución reconstituida debe administrarse por vía intravenosa y es para uso inmediato.
Los viales de 2 mg de alteplasa no están indicados para ser usados en esta indicación.
Población pediátrica
La experiencia con el uso de Actilyse en niños y adolescentes es limitada. Actilyse está contraindicado en el tratamiento del ictus isquémico agudo en niños y adolescentes menores de 16 años (ver Ficha técnica sección 4.3). La dosis en adolescentes entre 16 y 17 años es la misma que para los adultos (ver Ficha técnica sección 4.4 para ver las recomendaciones de las técnicas de imagen previas que se deben utilizar).
Los adolescentes de 16 años o mayores deben ser tratados de acuerdo con las instrucciones de uso del prospecto para la población adulta después de la obtención de imágenes mediante técnicas apropiadas para descartar falsos ictus y confirmar la oclusión arterial correspondiente al déficit neurológico.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ACTILYSE POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE Y PARA PERFUSIONForma farmacéutica: INYECTABLE, 1.500 UIPrincipio activo: apadamtase alfa and cinaxadamtase alfaFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 500 UIPrincipio activo: apadamtase alfa and cinaxadamtase alfaFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLEPrincipio activo: Proteina cFabricante: Takeda Manufacturing Austria AgRequiere receta
Médicos online para ACTILYSE POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE Y PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ACTILYSE POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE Y PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes