
ACICLOVIR BRILL PHARMA 30 MG/G POMADA OFTALMICA

Cómo usar ACICLOVIR BRILL PHARMA 30 MG/G POMADA OFTALMICA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Aciclovir Brill Pharma 30 mg/g pomada oftálmica
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Aciclovir Brill Pharma y para qué se utiliza
- Qué necesita saber antes de empezar a usar Aciclovir Brill Pharma
- Cómo usar Aciclovir Brill Pharma
- Posibles efectos adversos
- Conservación de Aciclovir Brill Pharma
- Contenido del envase e información adicional
1. Qué es Aciclovir Brill Pharma y para qué se utiliza
Este medicamento contiene aciclovir, un principio activo que pertenece al grupo de medicamentos llamados antivirales.
Está indicado para el tratamiento de la queratitis (inflamación de la córnea) causada por el virus herpes simplex.
2. Qué necesita saber antes de empezar a usar Aciclovir Brill Pharma
No use Aciclovir Brill Pharma
Si es alérgico al aciclovir, valaciclovir o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si piensa que esto le aplica, no use este medicamento hasta que lo consulte con su médico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento.
Puede aparecer un escozor leve y transitorio inmediatamente después de aplicarse este medicamento.
Tenga especial cuidado con este medicamento. Antes de usar Aciclovir Brill Pharma, su médico debe saber si usa lentes de contacto. Deje de usar lentes de contacto durante el tratamiento con este medicamento. Si no está seguro, consulte con su médico o farmacéutico antes de usar este medicamento.
Otros medicamentos y Aciclovir Brill Pharma
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluso los adquiridos sin receta.
Aunque no se han descrito interacciones de este medicamento mediante la administración oftálmica, consulte con su médico antes de usar otros medicamentos.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
Su médico valorará el beneficio para usted y el riesgo para su bebé de usar este medicamento durante el embarazo.
Lactancia
Aunque tras la administración tópica de este medicamento, la absorción sistémica es mínima, su médico valorará el beneficio para usted y el riesgo para su bebé de usar este medicamento durante la lactancia.
Conducción y uso de máquinas
Puede notar visión borrosa inmediatamente después de la aplicación de este medicamento. No conduzca ni utilice máquinas hasta que haya desaparecido este efecto.
3. Cómo usar Aciclovir Brill Pharma
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Adultos y niños:
- Aplicar aproximadamente 10 mm de Aciclovir Brill Pharma sobre el ojo infectado 5 veces al día.
- Se debe aplicar este medicamento a intervalos de 4 horas. Los horarios sugeridos son: 7 h, 11 h, 15 h, 19 h y 23 h.
- Su visión puede ser borrosa durante 5 o 10 minutos después de aplicar este medicamento.
- El tratamiento deberá continuarse durante al menos 3 días después de que el ojo haya mostrado mejoría.
Si está utilizando más de un medicamento por vía oftálmica, debe espaciar las aplicaciones de los medicamentos al menos 5 minutos o según le indique su médico. Las pomadas oftálmicas deben administrarse en último lugar.
Cómo aplicar Aciclovir Brill Pharma en el ojo
Este medicamento se debe administrar únicamente por vía oftálmica.
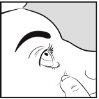
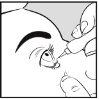

- Lávese las manos
- Use el dedo para bajar suavemente el párpado inferior del ojo infectado
- Incline la cabeza ligeramente hacia atrás y mire hacia arriba
- Aplique 10 mm de Aciclovir Brill Pharma en el lado interno del párpado inferior. Trate de evitar que la punta del tubo toque alguna parte del ojo.
- Mantenga el ojo cerrado durante 30 segundos.
- Lávese las manos después de aplicar la pomada.
Si usa más Aciclovir Brill Pharma del que debe
Si usa más Aciclovir Brill Pharma del que debe, retire el exceso cuidadosamente. No es probable que se produzcan cuadros de sobredosis con este medicamento.
No obstante, no se esperan efectos adversos en el caso de ingerir los 135 mg de aciclovir que contiene el envase.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Aciclovir Brill Pharma
En caso de olvidar una aplicación, realícela lo antes posible y continúe el tratamiento de la forma prescrita. Sin embargo, si es cercana a la hora de su próxima aplicación, omita la aplicación olvidada.
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Siinterrumpeel tratamiento con Aciclovir Brill Pharma
No interrumpa el tratamiento con Aciclovir Brill Pharma sin consultar con su médico.
Es importante que use este medicamento durante el tiempo indicado para eliminar completamente la infección.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10personas):
- Irritación o sensación de cuerpo extraño en el ojo (sensación de tener algo en el ojo)
- Efectos en la superficie de la córnea (capa transparente frente al iris y la pupila) que pueden causar cambios en la visión (visión borrosa) y/o sensibilidad incómoda a la luz.
No precisa la interrupción del tratamiento y cura sin secuelas aparentes.
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10personas):
- Escozor leve y transitorio del ojo inmediatamente después de la aplicación.
- Ojos rojos, llorosos y con picor (conjuntivitis).
Efectos adversos raros (pueden afectar hasta 1 de cada 1.000personas):
- Inflamación del párpado (blefaritis).
Efectos adversos muy raros (pueden afectar hasta 1 de cada 10.000personas):
- Reacciones de hipersensibilidad inmediata (reacciones alérgicas).
Los signos de las reacciones alérgicas pueden incluir:
- hinchazón de cara, labios, lengua u otras partes del cuerpo (angioedema).
- erupción, picor o urticaria en la piel.
- falta de aliento, dificultad respiratoria o sibilancias.
- fiebre inexplicable y sensación de desmayo, particularmente estando de pie.
Contacte con un médico inmediatamente si tiene alguno de estos síntomas. Deje de usar Aciclovir Brill Pharma.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Aciclovir Brill Pharma
Mantener fuera de la vista y del alcance de los niños.
Conservar por debajo de 25 ºC.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Desechar a las 4 semanas después de la primera apertura.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Aciclovir Brill Pharma
- El principio activo es aciclovir. Cada gramo de pomada contiene 30 mg de aciclovir.
- Los demás componentes (excipientes) son: vaselina blanca.
Aspecto del producto y contenido del envase
Tubo (aluminio) con tapón de rosca (polietileno) acondicionado en cajas de cartón. Cada caja contiene un tubo de 4,5 gramos de pomada.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
BRILL PHARMA, S.L.
C/ Munner, 8
08022 Barcelona
España
Responsable de la fabricación
URSAPHARM Arzneimittel GmbHIndustriestraße 3566129 Saarbrücken
Alemania
Fecha de la última revisión de este prospecto: Junio 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Precio medio en farmacia15.06 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ACICLOVIR BRILL PHARMA 30 MG/G POMADA OFTALMICAForma farmacéutica: POMADA OFTALMICA, 30 mg/gPrincipio activo: aciclovirFabricante: Agepha Pharma S.R.O.Requiere recetaForma farmacéutica: GEL OFTALMICO, 0,15%Principio activo: GanciclovirFabricante: Laboratoires TheaRequiere recetaForma farmacéutica: COLIRIO, 5 mg/mlPrincipio activo: moxifloxacinaFabricante: Tiedra Farmaceutica S.L.Requiere receta
Médicos online para ACICLOVIR BRILL PHARMA 30 MG/G POMADA OFTALMICA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ACICLOVIR BRILL PHARMA 30 MG/G POMADA OFTALMICA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















