
VISCOTEÍNA 50 mg/ml SOLUCIÓN ORAL

Cómo usar VISCOTEÍNA 50 mg/ml SOLUCIÓN ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO: INFORMACIÓN PARA EL USUARIO
Viscoteína 50 mg/ml solución oral
Carbocisteína
Lea todo el prospecto detenidamente porque contiene información importante para usted.
Este medicamento puede adquirirse sin receta. No obstante, para obtener los mejores resultados, debe utilizarse adecuadamente.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si los síntomas empeoran o persisten después de 5 días, o se produce: fiebre, erupciones en la piel, dolor de cabeza persistente o dolor de garganta, debe consultar a un médico.
- Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Contenido del prospecto:
- Qué es Viscoteína y para qué se utiliza
- Antes de tomar Viscoteína
- Cómo tomar Viscoteína
- Posibles efectos adversos
- Conservación de Viscoteína
- Información adicional
1. Qué es Viscoteína y para qué se utiliza
Viscoteína pertenece a un grupo de medicamentos llamados mucolíticos, y se utiliza para fluidificar las secreciones bronquiales excesivas y/o espesas, facilitando su eliminación.
2. Antes de tomar Viscoteína
No tome Viscoteína:
- Si es alérgico (hipersensible) a la carbocisteína y sus derivados, o a cualquiera de los demás componentes de Viscoteína.
- Si padece úlcera de estómago o duodeno.
- Este medicamento no debe administrarse a niños menores de 2 años.
- Si es asmático o padece una insuficiencia respiratoria grave.
Tenga especial cuidado con Viscoteína:
- Si padece insuficiencia hepática o renal grave
- En pacientes ancianos, que son especialmente sensibles a los efectos adversos de la carbocisteína.
- Durante los primeros días es normal que aumente la expectoración, pero si los síntomas persisten más de 5 días o empeoran, debe consultar a su médico.
Uso de otros medicamentos:
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
Consulte a su médico antes de tomar Viscoteína si está tomando medicamentos contra la tos o que disminuyen las secreciones bronquiales.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
No se debe tomar este medicamento durante el embarazo ni durante la lactancia.
IMPORTANTE PARA LA MUJER
Si está usted embarazada o cree que pudiera estarlo, consulte a su médico antes de tomar este medicamento. El consumo de medicamentos durante el embarazo puede ser peligroso para el embrión o el feto y debe ser vigilado por su médico.
Conducción y uso de máquinas
No se han descrito interferencias sobre la conducción y/o el uso de máquinas si se está tomando Viscoteína.
Información importante sobre algunos de los componentes de Viscoteína
Este medicamento contiene sacarosa. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Los pacientes con diabetes mellitus deben tener en cuenta que este medicamento contiene 1,5 g de sacarosa por cada 5 ml.
Puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de metilo, sal de sodio (E-219) y parahidroxibenzoato de propilo, sal de sodio (E-217).
Los pacientes con dietas pobres en sodio deben tener en cuenta que este medicamento contiene 34,75 mg de sodio por dosis de 5 ml y 104,24 mg de sodio por dosis de 15 ml.
3. Cómo tomar Viscoteína
Consulte a su médico o farmacéutico si tiene dudas.
Este medicamento se administra por vía oral, sólo o bien diluido en agua u otro líquido y preferentemente antes de las comidas. La dosis normal es:
- Adultos y niños mayores de 12 años: 15 ml (750 mg de carbocisteína) tres veces al día.
- Niños de 5 a 12 años: 5 ml (250 mg de carbocisteína) tres veces al día.
- Niños de 2 a 5 años: 2,5 ml (125 mg de carbocisteína) tres veces al día.
Se recomienda beber abundante líquido durante el día.
Instrucciones para la correcta administración del preparado
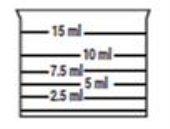
Con el fin de asegurar una dosificación correcta y dado su uso en pediatría, se recomienda realizar la dosificación utilizando el vaso dosificador incluido en la presentación que tiene marcadas las siguientes medidas: 2,5 ml, 5 ml, 7,5 ml, 10 ml y 15 ml.
Coloque el vaso dosificador sobre una superficie plana y a la altura de los ojos. Llénelo de la solución hasta la línea que indica su dosis.
Después de su uso, lave el vaso dosificador con agua.
Si los síntomas empeoran o persisten después de 5 días, o se produce: fiebre, erupciones en la piel, dolor de cabeza persistente o dolor de garganta, debe consultar a un médico.
Si toma más Viscoteína del que debiera:
La ingestión accidental de dosis muy elevadas puede producir reacciones de hipersensibilidad, náuseas, vómitos, diarreas, dolor de cabeza, hemorragias gastrointestinales, etc.
Si ha tomado más Viscoteína del que debiera, consulte inmediatamente a su médico o farmacéutico, o bien llame al Servicio de Información Toxicológica, teléfono: 91 562 0420, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Viscoteína:
No tome una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Viscoteína puede tener efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos observados se describen a continuación según la frecuencia de presentación: Muy frecuentes (que afecta a más de 1 de cada 10 pacientes), Frecuentes (que afecta a entre 1 y 10 de cada 100 pacientes), Poco frecuentes (que afecta a entre 1 y 10 de cada 1.000 pacientes), Raros (que afecta a entre 1 y 10 de cada 10.000 pacientes), Muy raros (que afecta a menos de 1 de cada 10.000 pacientes).
Efectos adversos frecuentes (que afecta a entre 1 y 10 de cada 100 pacientes):
Se pueden presentar molestias digestivas (malestar gástrico, náuseas, diarreas) sobre todo a dosis altas, que suelen desaparecer con la reducción de la dosis administrada.
Efectos adversos raros(que afecta a entre 1 y 10 de cada 10.000 pacientes):
Reacciones de hipersensibilidad (reacciones alérgicas), acompañadas de urticaria (aparición de habones y picor), dolor de cabeza, erupciones en la piel.
Efectos adversos muy raros (que afecta a menos de 1 de cada 10.000 pacientes):
Espasmo bronquial (sensación repentina de dificultad para respirar como ocurre en el asma). En estos casos se aconseja suspender el tratamiento y consultar al médico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Viscoteína
Mantener fuera del alcance y de la vista de los niños.
Conservar por debajo de 30ºC.
No utilice Viscoteína después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Información adicional
Composición de Viscoteína:
- El principio activo es carbocisteína. Cada ml de solución oral contiene 50 mg de carbocisteína.
- Los demás componentes son: sacarosa, sacarina sódica, parahidroxibenzoato de metilo, sal de sodio (E-219), parahidroxibenzoato de propilo, sal de sodio (E-217), colorante caramelo (E-150), aroma caramelo, hidróxido sódico para ajuste de pH, agua purificada.
Aspecto del producto y contenido del envase:
Viscoteína es una solución para administración por vía oral que se presenta en frasco de vidrio con tapón de polipropileno/polietileno de alta densidad (PP/HDPE) con cierre a prueba de niños (Child-proof), que contiene 200 ml de solución oral y un vasito dosificador.
El vasito dosificador tiene marcadas las siguientes medidas: 2,5 ml, 5 ml, 7,5 ml, 10 ml y 15 ml.
Titular de la autorización de comercialización
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
España
Responsable de la fabricación:
Faes Farma, S.A.
Parque Científico y Tecnológico de Bizkaia
Ibaizabal Bidea, Edificio 901
48160 Derio (Bizkaia)
España
Este prospecto ha sido aprobado en: Febrero 2025
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VISCOTEÍNA 50 mg/ml SOLUCIÓN ORALForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 2 g carbocisteina/ 100 mlPrincipio activo: CarbocisteinaFabricante: Almirall S.A.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 50 mg carbocisteína / mlPrincipio activo: CarbocisteinaFabricante: Laboratorios Cinfa S.A.No requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 750 mgPrincipio activo: CarbocisteinaFabricante: Laboratorios Cinfa S.A.No requiere receta
Médicos online para VISCOTEÍNA 50 mg/ml SOLUCIÓN ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VISCOTEÍNA 50 mg/ml SOLUCIÓN ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















