
IMBRUVICA 420 mg FILM-COATED TABLETS

How to use IMBRUVICA 420 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information: Summary of Product Characteristics
IMBRUVICA 140 mg film-coated tablets
IMBRUVICA 280 mg film-coated tablets
IMBRUVICA 420 mg film-coated tablets
IMBRUVICA 560 mg film-coated tablets
Ibrutinib
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is IMBRUVICA and what is it used for
- 2 What you need to know before you take IMBRUVICA
- How to take IMBRUVICA
- Possible side effects
- Storage of IMBRUVICA
- Contents of the pack and other information
1. What is IMBRUVICA and what is it used for
What is IMBRUVICA
IMBRUVICA is a cancer medicine that contains the active substance ibrutinib, which belongs to a class of medicines called kinase inhibitors.
What IMBRUVICA is used for
It is used in adults to treat the following blood cancers:
- Mantle cell lymphoma (MCL), a type of cancer that affects the lymph nodes, when the disease has come back or has not responded to treatment.
- Chronic lymphocytic leukemia (CLL), a type of cancer that affects the white blood cells called lymphocytes, which also affects the lymph nodes. IMBRUVICA is used in patients with CLL who have not been previously treated or when the disease has come back or has not responded to treatment.
- Waldenström's macroglobulinemia (WM), a type of cancer that affects the white blood cells called lymphocytes. It is used when the disease has come back or has not responded to treatment or in patients for whom chemotherapy given with an antibody is not a suitable treatment.
How IMBRUVICA works
In MCL, CLL, and WM, IMBRUVICA works by blocking Bruton's tyrosine kinase, a protein in the body that helps cancer cells grow and survive. By blocking this protein, IMBRUVICA helps destroy and reduce the number of cancer cells. It may also slow down the worsening of the cancer.
2. What you need to know before you take IMBRUVICA
Do not take IMBRUVICA
- if you are allergic to ibrutinib or any of the other ingredients of this medicine (listed in section 6)
- if you are taking a medicine containing a plant called St. John's Wort (Hypericum perforatum), used for depression. If you are not sure, consult your doctor, pharmacist, or nurse before taking this medicine.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to take IMBRUVICA:
- if you have ever had unusual bleeding or bruising or if you are taking any medicines or supplements that increase your risk of bleeding (see section “Using IMBRUVICA with other medicines”)
- if you have had irregular heartbeats, have a history of irregular heartbeats or severe heart failure, or if you feel any of the following: difficulty breathing, weakness, dizziness, fainting, or feeling like you are about to faint, chest pain, or swollen legs
- if you have liver or kidney problems
- if you have high blood pressure
- if you have recently undergone surgery, especially if it has affected the absorption of food or medicines in the stomach or intestine
- if you are going to undergo surgery, your doctor may ask you to stop taking IMBRUVICA for a short period (3 to 7 days) before and after surgery
- if you have had a hepatitis B infection or may have it now. This is because IMBRUVICA may reactivate hepatitis B. Patients will be carefully monitored by their doctor for signs of this infection before starting treatment.
If any of the above apply to you (or if you are not sure), consult your doctor, pharmacist, or nurse before taking this medicine.
When taking IMBRUVICA, inform your doctor immediately if you notice or someone notices in you: memory loss, confusion, difficulty walking, or vision loss – these may be due to a rare but serious brain infection that can be fatal (Progressive Multifocal Leukoencephalopathy or PML).
Tests and checks before and during treatment
Tumor lysis syndrome (TLS): There have been cases of abnormal levels of chemicals in the blood caused by the rapid breakdown of cancer cells during treatment, and sometimes even without treatment. This can lead to changes in kidney function, abnormal heart rhythm, or seizures. Your doctor or other healthcare professional may perform blood tests to check for TLS.
Lymphocytosis: Laboratory tests may show an increase in white blood cells (called "lymphocytes") in your blood during the first few weeks of treatment. This effect is expected and may last for several months. This does not necessarily mean that your blood cancer is getting worse.
Your doctor will check your blood tests before or during treatment, and in rare cases, may need to give you another medicine. Talk to your doctor about the meaning of the results of these tests.
Children and adolescents
IMBRUVICA should not be used in children or adolescents, as it has not been studied in these age groups.
Other medicines and IMBRUVICA
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription, herbal medicines, and supplements. This is because IMBRUVICA may affect the way other medicines work. Also, other medicines may affect the way IMBRUVICA works.
IMBRUVICA may make you bleed more easily.This means you should tell your doctor if you are taking other medicines that increase your risk of bleeding. These include:
- aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen or naproxen
- anticoagulants such as warfarin, heparin, or other medicines that prevent blood clots
- supplements that may increase your risk of bleeding, such as fish oil, vitamin E, or flaxseed.
If any of the above apply to you (or if you are not sure), consult your doctor, pharmacist, or nurse before taking IMBRUVICA.
Tell your doctor if you are taking any of the following medicines: The effects of IMBRUVICA or other medicines may be affected if you take IMBRUVICA with any of the following medicines:
- antibiotics to treat bacterial infections: clarithromycin, telithromycin, ciprofloxacin, erythromycin, or rifampicin
- medicines for fungal infections: posaconazole, ketoconazole, itraconazole, fluconazole, or voriconazole
- medicines for HIV infection: ritonavir, cobicistat, indinavir, nelfinavir, saquinavir, amprenavir, atazanavir, or fosamprenavir
- medicines to prevent nausea and vomiting associated with chemotherapy: aprepitant
- medicines for depression: nefazodone
- medicines called kinase inhibitors for the treatment of other cancers: crizotinib or imatinib
- medicines called calcium channel blockers for high blood pressure or chest pain: diltiazem or verapamil
- medicines called statins for high cholesterol: rosuvastatin
- medicines for the heart/antiarrhythmics: amiodarone or dronedarone
- medicines to prevent seizures or to treat epilepsy, or medicines to treat a painful condition of the face called trigeminal neuralgia: carbamazepine or phenytoin.
If any of the above apply to you (or if you are not sure), consult your doctor, pharmacist, or nurse before taking IMBRUVICA.
If you are taking digoxin, a medicine used for heart problems, or methotrexate, a medicine used to treat other cancers and to reduce the activity of the immune system (e.g., for rheumatoid arthritis or psoriasis), you should take it at least 6 hours before or after IMBRUVICA.
Using IMBRUVICA with food
Do not take IMBRUVICA with grapefruit or Seville oranges: this means you cannot eat them, drink their juice, or take a supplement that may contain them. This is because they can increase the amount of IMBRUVICA in your blood.
Pregnancy and breastfeeding
You should not become pregnant while taking this medicine.
IMBRUVICA should not be used during pregnancy. There is no information on the safety of IMBRUVICA in pregnant women.
Women of childbearing potential must use a very effective method of contraception during treatment and for up to 3 months after receiving IMBRUVICA, to prevent pregnancy during treatment with IMBRUVICA. If you use hormonal contraceptives, such as the pill or contraceptive devices, you should also use a barrier contraceptive method (e.g., condoms).
- Tell your doctor immediately if you become pregnant.
- Do not breastfeed while taking this medicine.
Driving and using machines
You may feel tired or dizzy after taking IMBRUVICA, which may affect your ability to drive or use any tools or machines.
IMBRUVICA contains lactose
IMBRUVICA contains lactose (a type of sugar). If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
IMBRUVICA contains sodium
IMBRUVICA contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
3. How to take IMBRUVICA
Follow exactly the instructions of your doctor, pharmacist, or nurse for taking this medicine. If you are not sure, consult your doctor, pharmacist, or nurse again.
How much to take
Mantle cell lymphoma (MCL)
The recommended dose of IMBRUVICA is 560 mg once a day.
Chronic lymphocytic leukemia (CLL)/Waldenström's macroglobulinemia (WM)
The recommended dose of IMBRUVICA is 420 mg once a day.
Your doctor may need to adjust your dose.
How to take IMBRUVICA
- Take the tablets by mouth with a glass of water.
- Take the tablets at about the same time every day.
- Swallow the tablets whole. Do not break or chew the tablets.
If you take more IMBRUVICA than you should
If you take more IMBRUVICA than you should, consult your doctor or go to the hospital immediately.
Take the tablets and this leaflet with you.
If you forget to take IMBRUVICA
- If you forget a dose, you can take it as soon as possible on the same day and return to your normal schedule the next day.
- Do not take a double dose to make up for a forgotten dose.
- If you are not sure, talk to your doctor, pharmacist, or nurse about when to take your next dose.
If you stop taking IMBRUVICA
Do not stop taking this medicine unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur with this medicine:
Stop taking IMBRUVICA and tell your doctor immediately if you experience any of the following side effects:
rash with blisters and itching, difficulty breathing, swelling of the face, lips, tongue, or throat: you may be having an allergic reaction to the medicine.
Tell your doctor immediately if you experience any of the following side effects:
Very common(may affect more than 1 in 10 people)
- fever, chills, body pain, feeling tired, cold or flu symptoms, difficulty breathing: all these can be symptoms of an infection (caused by viruses, bacteria, or fungi). They can include infections of the nose, sinuses, or throat (upper respiratory tract infections), or of the lungs, or of the skin.
- bruises or increased tendency to bruise
- mouth sores
- headache
- constipation
- feeling or being sick (nausea or vomiting)
- diarrhea, your doctor may need to give you treatment to replace fluids and salts or another medicine
- rash
- pain in arms or legs
- back pain or joint pain
- muscle cramps, muscle pain, or muscle spasms
- low number of platelets, very low number of white blood cells: shown in blood tests
- swollen hands, ankles, or feet
- high blood pressure.
Common(may affect up to 1 in 10 people)
- severe infections that spread throughout the body (septicemia)
- urinary tract infections
- nosebleeds, small red or purple spots caused by bleeding under the skin
- bleeding in the stomach, intestine, stools, or urine, increased bleeding during menstruation, or bleeding from a wound that cannot be stopped
- increased heart rate, missed heartbeats, weak or irregular pulse, dizziness, difficulty breathing, chest discomfort (symptoms of heart rhythm disorders)
- increased number or proportion of white blood cells in blood tests
- low white blood cell count with fever (febrile neutropenia)
- abnormal levels of chemicals in the blood, caused by the rapid breakdown of cancer cells during treatment, and sometimes even without treatment (tumor lysis syndrome)
- skin cancer other than melanoma, most commonly basal cell carcinoma and squamous cell carcinoma
- feeling dizzy
- blurred vision
- redness of the skin
- high levels of "uric acid" in the blood (shown in blood tests), which can cause gout
- inflammation of the airways (pulmonary) that can lead to permanent damage
- breaking of the nails
- weakness, numbness, tingling, or pain in the hands or feet or other parts of the body (peripheral neuropathy).
Uncommon(may affect up to 1 in 100 people)
- confusion, headache with problems speaking or feeling like you are about to faint: these can be symptoms of a severe internal bleed in the brain
- allergic reaction, sometimes severe, which can include swelling of the face, lip, mouth, tongue, or throat, difficulty swallowing or breathing, rash with itching (urticaria)
- inflammation of the fatty tissue under the skin.
Rare(may affect up to 1 in 1,000 people)
- severe increase in white blood cell count that can cause cells to clump together.
Not known(frequency cannot be estimated from the available data)
- liver failure
- Severe skin rash with blisters and peeling of the skin, particularly around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of IMBRUVICA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP.
The expiry date is the last day of the month stated.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of IMBRUVICA
- The active ingredient is ibrutinib.
- IMBRUVICA 140 mg film-coated tablets: Each tablet contains 140 mg of ibrutinib.
- IMBRUVICA 280 mg film-coated tablets: Each tablet contains 280 mg of ibrutinib.
- IMBRUVICA 420 mg film-coated tablets: Each tablet contains 420 mg of ibrutinib.
- IMBRUVICA 560 mg film-coated tablets: Each tablet contains 560 mg of ibrutinib.
- The other components are:
- Tablet core: anhydrous colloidal silica, sodium croscarmellose, lactose monohydrate (see section 2 "IMBRUVICA contains lactose"), magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate (E487).
- Film coating: polyvinyl alcohol, macrogol, talc, titanium dioxide (E171);
IMBRUVICA 280 mg film-coated tablets also contain black iron oxide (E172) and red iron oxide (E172);
IMBRUVICA 560 mg film-coated tablets also contain red iron oxide (E172) and yellow iron oxide (E172).
Appearance and Container Content
IMBRUVICA 140 mg film-coated tablets
Greenish-yellow to green, round tablet (9 mm), marked with "ibr" on one side and "140" on the other side. Each 28-day box contains 28 film-coated tablets in 2 cardboard boxes with 14 film-coated tablets each. Each 30-day box contains 30 film-coated tablets in 3 cardboard boxes with 10 film-coated tablets each.
IMBRUVICA 280 mg film-coated tablets
Purple, oblong tablet (15 mm in length and 7 mm in thickness), marked with "ibr" on one side and "280" on the other side. Each 28-day box contains 28 film-coated tablets in 2 cardboard boxes with 14 film-coated tablets each. Each 30-day box contains 30 film-coated tablets in 3 cardboard boxes with 10 film-coated tablets each.
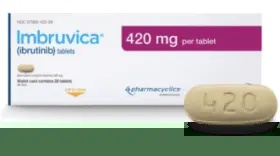
IMBRUVICA 420 mg film-coated tablets
Greenish-yellow to green, oblong tablet (17.5 mm in length and 7.4 mm in thickness), marked with "ibr" on one side and "420" on the other side. Each 28-day box contains 28 film-coated tablets in 2 cardboard boxes with 14 film-coated tablets each. Each 30-day box contains 30 film-coated tablets in 3 cardboard boxes with 10 film-coated tablets each.
IMBRUVICA 560 mg film-coated tablets
Yellow to orange, oblong tablet (19 mm in length and 8.1 mm in thickness), marked with "ibr" on one side and "560" on the other side. Each 28-day box contains 28 film-coated tablets in 2 cardboard boxes with 14 film-coated tablets each. Each 30-day box contains 30 film-coated tablets in 3 cardboard boxes with 10 film-coated tablets each.
Marketing Authorization Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen-Cilag SpA
Via C. Janssen,
Loc. Borgo S. Michele,
04100 Latina,
Italy
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lithuania UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
Bulgaria "JOHNSON & JOHNSON" EAD Tel: +359 2 489 94 00 | Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Czech Republic Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Hungary Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Denmark Janssen-Cilag A/S Tlf: +45 45 94 82 82 | Malta AM MANGION LTD. Tel: +356 2397 6000 |
Germany Janssen-Cilag GmbH Tel: +49 2137 955 955 | Netherlands Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Estonia UAB "JOHNSON & JOHNSON" Estonian branch Tel: +372 617 7410 | Norway Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Greece Janssen-Cilag Φαρμακευτική Α.Ε.Β.Ε. Tηλ: +30 210 80 90 000 | Austria Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
Spain Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Poland Janssen-Cilag Polska Sp. z o.o. Tel.:+48 22 237 60 00 |
France Janssen-Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Croatia Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | Romania Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenia Johnson & Johnson d.o.o. Tel.: +386 1 401 18 00 |
Iceland Janssen-Cilag AB c/o Vistor hf Sími: +354 535 7000 | Slovakia Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italy Janssen-Cilag SpA Tel: 800 688 777 / +39 02 2510 1 | Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
Cyprus Βαρνάβας Χατζηπαναγής Λτδ Τηλ: +357 22 207 700 | Sweden Janssen-Cilag AB Tel: +46 8 626 50 00 |
Latvia UAB "JOHNSON & JOHNSON" Latvian branch Tel: +371 678 93561 | United Kingdom Janssen-Cilag Ltd. Tel: +44 1 494 567 444 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to IMBRUVICA 420 mg FILM-COATED TABLETSDosage form: CAPSULE, 140 mgActive substance: ibrutinibManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: CAPSULE, 140 mgActive substance: ibrutinibManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: TABLET, 140 mgActive substance: ibrutinibManufacturer: Janssen-Cilag International N.VPrescription required
Online doctors for IMBRUVICA 420 mg FILM-COATED TABLETS
Discuss questions about IMBRUVICA 420 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






