EYLEA 114,3 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar EYLEA 114,3 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Eylea114,3mg/ml solución inyectable en jeringa precargada
aflibercept
Lea todo el prospecto detenidamente antes de que le administren este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Si experimenta efectos adversos, consulte a su médico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Eylea y para qué se utiliza
- Qué necesita saber antes de que le administren Eylea
- Cómo se administrará Eylea
- Posibles efectos adversos
- Conservación de Eylea
- Contenido del envase e información adicional
1. Qué es Eylea y para qué se utiliza
Qué es Eylea
Eylea contiene el principio activo aflibercept. Pertenece a un grupo de medicamentos llamados agentes antineovascularización.
Su médico le inyectará Eylea en el ojo para tratar unos trastornos oculares en pacientes adultos denominados:
- degeneración macular asociada a la edad (DMAE exudativa)
- alteración de la visión debida al edema macular diabético (EMD).
Estos trastornos afectan a la mácula. La mácula es la parte central de la membrana sensible a la luz que se encuentra en la parte posterior del ojo. Es la responsable de tener una visión clara.
La DMAE exudativa se produce cuando se forman y crecen vasos sanguíneos anómalos por debajo de la mácula. Los vasos sanguíneos anómalos pueden presentar fugas de líquido o de sangre hacia el interior del ojo. Los vasos sanguíneos con fugas que causan una hinchazón de la mácula provocan el EMD. Ambos trastornos pueden afectar a su visión.
Cómo funciona Eylea
Eylea detiene el crecimiento de los nuevos vasos sanguíneos anómalos en el ojo. Eylea puede ayudar a estabilizar y, en muchas ocasiones, a mejorar la visión.
2. Qué necesita saber antes de que le administren Eylea
No se le administrará Eylea si
- es alérgico a aflibercept o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- tiene una infección en el ojo o a su alrededor
- tiene dolor o enrojecimiento en el ojo (una inflamación grave del ojo).
Advertencias y precauciones
Consulte a su médico antes de que le administrenEylea si:
- sufre glaucoma, una enfermedad de los ojos causada por una presión elevada en el ojo
- tiene antecedentes de visión de destellos de luz o manchas flotantes oscuras y si su tamaño o número aumenta de repente
- le han operado de un ojo en las últimas 4 semanas o tiene programada una cirugía ocular en las 4 semanas siguientes.
Informe a su médico inmediatamente sipresenta:
- enrojecimiento del ojo
- dolor en el ojo
- aumento de las molestias en el ojo
- visión borrosa o disminuida
- aumento de la sensibilidad a la luz
Estos pueden ser síntomas de una inflamación o infección y su médico puede interrumpir el tratamiento con Eylea.
Además, es importante que sepa que:
- la seguridad y eficacia de Eylea cuando se administra en ambos ojos a la vez no se han estudiado y dicho uso puede aumentar el riesgo de que se produzcan efectos adversos.
- las inyecciones de Eylea pueden producir un aumento de la presión en el ojo en algunos pacientes en los 60 minutos siguientes a la inyección. Su médico le realizará un seguimiento después de cada inyección.
- su médico comprobará otros factores de riesgo que puedan aumentar la posibilidad de que se produzca un desgarro o un desprendimiento de las capas posteriores del ojo. En dichos casos, su médico le administrará Eylea con precaución.
- las mujeres en edad fértil deben utilizar métodos anticonceptivos efectivos durante el tratamiento y durante al menos 4 meses después de la última inyección de Eylea.
El uso de sustancias parecidas a las que contiene Eylea está potencialmente relacionado con el riesgo de bloqueo de los vasos sanguíneos por coágulos de sangre, que pueden dar lugar a un infarto de miocardio o un accidente cerebrovascular. Teóricamente, esto también podría ocurrir tras una inyección de Eylea en el ojo. Si ha sufrido un accidente cerebrovascular, un accidente cerebrovascular transitorio, o bien un infarto de miocardio en los últimos 6 meses, su médico administrará Eylea con precaución.
Niños y adolescentes
No se ha estudiado el uso de Eylea en niños y adolescentes menores de 18 años, porque las enfermedades indicadas ocurren principalmente en adultos. Por tanto, no procede su uso en este grupo de edad.
Otros medicamentos y Eylea
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
- Las mujeres que puedan quedarse embarazadas deben utilizar métodos de control del embarazo efectivos durante el tratamiento y durante al menos 4 meses después de la última inyección de Eylea.
- Hay experiencia limitada con el uso de Eylea en mujeres embarazadas. Las mujeres no deben recibir Eylea durante el embarazo a menos que el beneficio potencial para la mujer supere al riesgo potencial para el feto.
- Cantidades pequeñas de Eylea pueden pasar a la leche materna. Se desconoce el efecto en recién nacidos/lactantes alimentados con leche materna. No se recomienda el uso de Eylea durante la lactancia.
Por lo tanto, si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de recibir este medicamento.
Conducción y uso de máquinas
Después de la inyección de Eylea puede experimentar algunos problemas de visión transitorios. No conduzca ni use máquinas mientras duren estos problemas.
Eylea contiene polisorbato 20
Este medicamento contiene 0,021 mg de polisorbato 20 en cada dosis de 0,07 ml equivalente a 0,3 mg/ml.
Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
3. Cómo se administrará Eylea
La dosis recomendada es de 8 mg de aflibercept por inyección.
- Usted recibirá 1 inyección cada mes durante los primeros 3 meses.
- Después de esto, puede recibir inyecciones hasta cada 6 meses. Su médico decidirá la frecuencia según el estado de su ojo.
- Si su médico cambia su tratamiento a Eylea 114,3 mg/ml, su médico decidirá la frecuencia después de la primera inyección.
Forma de administración
Su médico le inyectará Eylea en el interior del ojo (inyección intravítrea).
Antes de la inyección, su médico utilizará un lavado ocular desinfectante para limpiar cuidadosamente su ojo para prevenir una infección. Su médico le administrará un colirio (anestésico local) para adormecer el ojo con el fin de reducir o prevenir el dolor de la inyección.
Si no se le ha administrado una dosis de Eylea
Pida una nueva cita con su médico lo antes posible.
Antes de interrumpir el tratamiento con Eylea
Hable con su médico antes de interrumpir el tratamiento. La interrupción del tratamiento puede aumentar el riesgo de pérdida de visión y su visión puede empeorar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos de la inyección de Eylea son debidos al propio medicamento o al procedimiento de inyección y en su mayoría afectan al ojo.
Algunos efectos adversos pueden sergraves
Póngase en contacto con su médico inmediatamente si presenta cualquiera de los siguientes trastornos:
- efecto adverso frecuente, que puede afectar hasta 1 de cada 10 personas
- enturbamiento del cristalino (catarata)
- sangrado en la parte posterior del ojo (hemorragia retiniana)
- aumento de la presión en el interior del ojo
- sangrado en el interior del ojo (hemorragia vítrea)
- efecto adverso no frecuente, que puede afectar hasta 1 de cada 100 personas
- ciertas formas de enturbamiento del cristalino (catarata subcapsular/nuclear)
- desprendimiento, desgarro o hemorragia de la capa sensible a la luz en la parte posterior del ojo, que producen destellos de luz con manchas flotantes que en ocasiones progresa a pérdida de visión (desgarro o desprendimiento de la retina)
Otros posibles efectos adversos
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- reacciones alérgicas
- manchas en la visión (partículas flotantes en el humor vítreo)
- desprendimiento de la sustancia similar a un gel que se encuentra en el interior del ojo (desprendimiento de vítreo)
- disminución de la agudeza visual
- dolor ocular
- sangrado en el interior del ojo (hemorragia conjuntival)
- daños en la capa transparente del globo ocular que se encuentra delante del iris (queratitis punteada, abrasión corneal)
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- desprendimiento o desgarro de una de las capas de la parte posterior del ojo que producen destellos de luz con manchas flotantes que en ocasiones progresa a pérdida de visión (desgarro/desprendimiento del epitelio pigmentario de la retina)
- inflamación del iris, de otras partes del ojo o de la sustancia similar a un gel que se encuentra en el interior del ojo (uveítis, iritis, iridociclitis, vitritis)
- ciertas formas de enturbiamiento del cristalino (catarata cortical)
- daños en la capa superficial del globo ocular (erosión de la córnea)
- visión borrosa
- dolor en el lugar de inyección
- sensación de tener algo dentro del ojo
- aumento de la producción de lágrimas
- sangrado en el lugar de inyección
- enrojecimiento del ojo
- hinchazón del párpado
- enrojecimiento del ojo (hiperemia ocular)
- irritación en el lugar de inyección
Raros(pueden afectar hasta 1 de cada 1 000 personas):
- hinchazón de la capa superficial del globo ocular (edema corneal)
- enturbiamiento del cristalino (opacidad lenticular)
- degeneración de la membrana sensible a la luz que se encuentra en la parte posterior del ojo (degeneración retiniana)
- irritación del párpado
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- inflamación de la parte blanca del ojo asociada con enrojecimiento y dolor (escleritis)
Además de los previamente mencionados, se pueden producir los siguientes efectos adversos:
- sensación anormal en el ojo
- daños en la superficie de la parte delantera transparente del ojo (defecto en el epitelio corneal)
- inflamación de otras partes del ojo (células flotantes en la cámara anterior)
- inflamación o infección grave dentro del ojo (endoftalmitis)
- ceguera
- enturbiamiento del cristalino debido a lesión (catarata traumática)
- pus en el ojo (hipopión)
- reacciones alérgicas graves
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Eylea
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta después de “CAD/EXP”. La fecha de caducidad es el último día del mes que se indica.
- Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
- Conservar la jeringa precargada en su blíster y en el embalaje exterior para protegerla de la luz.
- Antes de usar, el blíster sin abrir de Eylea puede conservarse fuera de la nevera por debajo de 25 ºC durante un máximo de 24 horas.
6. Contenido del envase e información adicional
Composición de Eylea
- El principio activo es aflibercept. 1 ml de solución contiene 114,3 mg de aflibercept. Cada jeringa precargada contiene 0,184 ml. Esto proporciona una cantidad utilizable para administrar una dosis única de 0,07 ml que contiene 8 mg de aflibercept.
- Los demás componentes son: sacarosa, clorhidrato de arginina, clorhidrato de histidina monohidrato, histidina, polisorbato 20, agua para preparaciones inyectables.
Ver “Eylea contiene polisorbato 20” en la sección 2 para más información.
Aspecto de Eylea y contenido del envase
Eylea 114,3 mg/ml solución inyectable en jeringa precargada es una solución inyectable (inyectable). La solución es de incolora a amarillo pálido.
Envase: 1 jeringa precargada.
Titular de la autorización de comercialización
Bayer AG
51368 Leverkusen
Alemania
Responsable de la fabricación
Bayer AG
Müllerstraße 178
13353 Berlin
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 | Lietuva UAB Bayer Tel. +37 05 23 36 868 |
| Luxembourg/Luxemburg Bayer SA-NV Tél/Tel: +32-(0)2-535 63 11 |
Ceská republika Bayer s.r.o. Tel: +420 266 101 111 | Magyarország Bayer Hungária KFT Tel:+36 14 87-41 00 |
Danmark Bayer A/S Tlf: +45 45 23 50 00 | Malta Alfred Gera and Sons Ltd. Tel: +35 621 44 62 05 |
Deutschland Bayer Vital GmbH Tel: +49 (0)214-30 513 48 | Nederland Bayer B.V. Tel: +31-23 – 799 1000 |
Eesti Bayer OÜ Tel: +372 655 8565 | Norge Bayer AS Tlf: +47 23 13 05 00 |
Ελλ?δα Bayer Ελλ?ς ΑΒΕΕ Τηλ: +30-210-61 87 500 | Österreich Bayer Austria Ges.m.b.H. Tel: +43-(0)1-711 46-0 |
España Bayer Hispania S.L. Tel: +34-93-495 65 00 | Polska Bayer Sp. z o.o. Tel: +48 22 572 35 00 |
France Bayer HealthCare Tél (N° vert): +33-(0)800 87 54 54 | Portugal Bayer Portugal, Lda. Tel: +351 21 416 42 00 |
Hrvatska Bayer d.o.o. Tel: +385-(0)1-6599 900 | România SC Bayer SRL Tel: +40 21 529 59 00 |
Ireland Bayer Limited Tel: +353 1 216 3300 | Slovenija Bayer d. o. o. Tel: +386 (0)1 58 14 400 |
Ísland Icepharma hf. Sími: +354 540 8000 | Slovenská republika Bayer spol. s r.o. Tel. +421 2 59 21 31 11 |
Italia Bayer S.p.A. Tel: +39 02 397 8 1 | Suomi/Finland Bayer Oy Puh/Tel: +358- 20 785 21 |
Κ?προς NOVAGEM Limited Tηλ: +357 22 48 38 58 | Sverige Bayer AB Tel: +46 (0) 8 580 223 00 |
Latvija SIA Bayer Tel: +371 67 84 55 63 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
Si desea información local, escanee aquí para acceder al sitio web https://www.pi.bayer.com/eylea4.
Se incluye un código QR con el enlace al prospecto.
--------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales sanitarios:
La jeringa precargada con sistema de dosificación OcuClick es para un solo uso en un único ojo. La extracción de múltiples dosis de una única jeringa precargada con sistema de dosificación OcuClick puede aumentar el riesgo de contaminación y posterior infección.
Noutilizar si el embalaje o sus componentes han caducado, presentan daños o han sido manipulados.
Comprobar la etiqueta de la jeringa precargada con sistema de dosificación OcuClick para asegurarse de tener la dosis de Eylea que tenía previsto utilizar. La dosis de 8 mg requiere el uso de la jeringa precargada de Eylea 114,3 mg/ml.
La inyección intravítrea debe realizarse con una aguja de inyección de 30 G × ½ pulgada (1,27 cm) (no incluida).
El uso de una aguja de menor tamaño (mayor calibre) que la aguja de inyección recomendada de 30 G × ½ pulgada (1,27 cm) puede provocar un aumento de la fuerza de inyección.
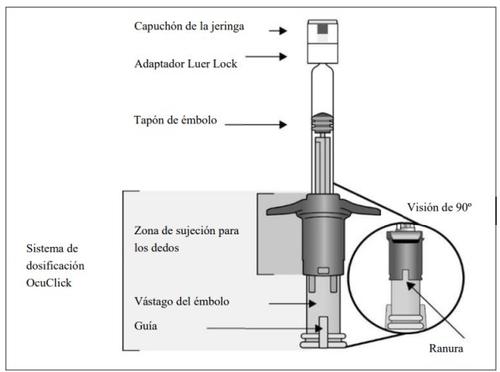
Descripción de la jeringa precargada con sistema de dosificación OcuClick integrado | ||
| ||
1. | Preparar | |
Cuando esté preparado para administrar Eylea 114,3 mg/ml, abra la caja y extraiga el blíster esterilizado. Despegue cuidadosamente la lámina del blíster para garantizar la esterilidad de su contenido. Mantenga la jeringa en la bandeja estéril hasta que esté preparado para acoplar la aguja de inyección. Utilizar una técnica aséptica para la realización de los pasos 2 a 9. | ||
2. | Extraer la jeringa | |
Extraer la jeringa del blíster esterilizado. | ||
3. | Inspeccionar la jeringa y la solución inyectable | |
Noutilizar la jeringa precargada si
| ||
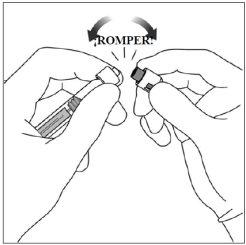
4. | Romper el capuchón de la jeringa | |
Para romper(no girar) el capuchón de la jeringa, sostener la jeringa en una mano y el capuchón de la jeringa con el pulgar y el índice de la otra mano. Nota:No tirar del vástago del émbolo hacia atrás. |
| |
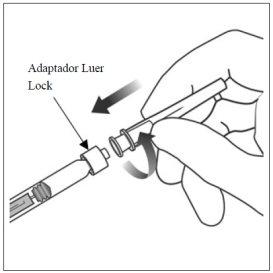
5. | Acoplar la aguja | |
Encajar firmemente la aguja de inyección de 30 G × ½ pulgada (1,27 cm) en la punta de la jeringa con el adaptador Luer Lock realizando un movimiento giratorio. |
| |
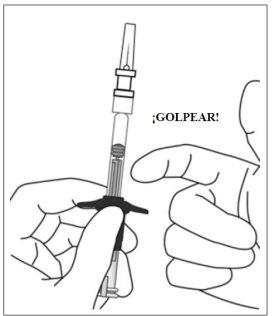
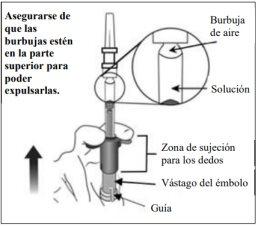
6. | Eliminar las burbujas de aire | |
Mantener la jeringa con la aguja apuntando hacia arriba y comprobar que no hay burbujas en su interior. Si las hay, golpear suavemente la jeringa con el dedo hasta que estas asciendan a su parte superior. |
| |
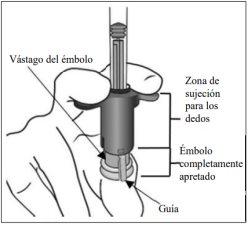
7. | Expulsar el aire y el exceso de volumen para purgar | |
La jeringa no tiene una línea de dosificación porque está diseñada para que la dosis se ajuste mecánicamente, tal como se explica en los pasos descritos a continuación. El purgado y el ajuste de la dosis se deben realizar conforme a los pasos siguientes. Para eliminar todas las burbujas y expulsar el exceso de medicamento, apretar lentamente el vástago del émbolo (imagen inferior izquierda) hasta que se detenga, es decir, cuando la guía del vástago del émbolo alcance la zona de sujeción para los dedos (imagen inferior derecha). | ||
|
| |
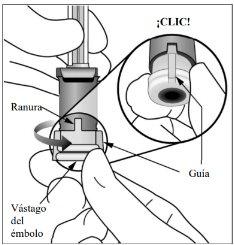
8. | Ajustar la dosis | |
Girar el extremo del vástago del émbolo 90 grados en sentido horario o antihorario hasta que la guía del vástago del émbolo se alinee con la ranura. Puede que se oiga un “clic”. Nota:Ahora el dispositivo está listo para la administración de la dosis. No empuje el vástago del émbolo antes de su inserción en el ojo. |
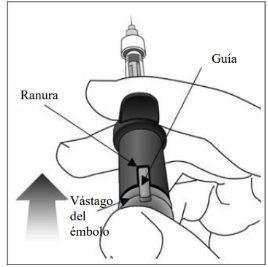
| |
9. | Administrar la inyección | |
Insertar la aguja en el sitio de inyección ocular. Inyectar la solución empujando el vástago del émbolo hacia dentro hasta que se detenga, es decir, hasta que la guía se encuentre completamente dentro de la ranura. No aplicar presión adicional una vez que la guía se encuentre dentro de la ranura. Es normal que quede una pequeña cantidad de solución residual en la jeringa. |
| |
10. | La jeringa precargada es para la administración de una sola dosis y para un solo uso. Tras la inyección, desechar la jeringa usada en un recipiente para objetos punzantes. |
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EYLEA 114,3 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 40 mg/mlPrincipio activo: AfliberceptFabricante: Sandoz GmbhRequiere recetaForma farmacéutica: INYECTABLE, 40 mg/mlPrincipio activo: AfliberceptFabricante: Sandoz GmbhRequiere recetaForma farmacéutica: INYECTABLE, 40 mg/mlPrincipio activo: AfliberceptFabricante: Celltrion Healthcare Hungary Kft.Requiere receta
Médicos online para EYLEA 114,3 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EYLEA 114,3 MG/ML SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes