
DUODOPA 240 MG/ML + 12 MG/ML SOLUCION PARA PERFUSION


Cómo usar DUODOPA 240 MG/ML + 12 MG/ML SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Duodopa solución para perfusión y para qué se utiliza
- Qué necesita saber antes de empezar a usar Duodopa solución para perfusión
- Cómo usar Duodopa solución para perfusión
- Posibles efectos adversos
- Conservación de Duodopa solución para perfusión
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Duodopa 240mg/ml + 12mg/ml solución para perfusión
foslevodopa/foscarbidopa
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Duodopa solución para perfusión y para qué se utiliza
- Qué necesita saber antes de empezar a usar Duodopa solución para perfusión
- Cómo usar Duodopa solución para perfusión
- Posibles efectos adversos
- Conservación de Duodopa solución para perfusión
- Contenido del envase e información adicional
- Instrucciones de uso de Duodopa solución para perfusión con la bomba Vyafuser
1. Qué es Duodopa solución para perfusión y para qué se utiliza
Este medicamento contiene dos principios activos, foslevodopa y foscarbidopa, y se utiliza para tratar la enfermedad de Parkinson.
Cómo funciona Duodopa solución para perfusión
- Foslevodopa se transforma en “dopamina” en el organismo. Esta se suma a la dopamina que está ya presente en su cerebro y en su médula espinal. La dopamina ayuda a transmitir las señales entre las células nerviosas.
- Los niveles bajos de dopamina son la causa de los síntomas de la enfermedad de Parkinson, como temblor, sensación de rigidez, movimiento lento y trastornos de equilibrio.
- El tratamiento con foslevodopa aumenta la cantidad de dopamina en su cuerpo. Lo que significa que reduce estos síntomas.
- La foscarbidopa mejora el efecto de la foslevodopa. También reduce los efectos adversos de la foslevodopa.
2. Qué necesita saber antes de empezar a usar Duodopa solución para perfusión
No use Duodopa solución para perfusión si
- es alérgico a foslevodopa, foscarbidopa o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- tiene un trastorno ocular llamado “glaucoma de ángulo estrecho”
- padece problemas del corazón graves
- padece un latido irregular grave del corazón (arritmia)
- padece un accidente cerebrovascular agudo
- está tomando medicamentos para la depresión denominados inhibidores selectivos de la MAO‑A e inhibidores no selectivos de la MAO como moclobemida y fenelzina. Debe interrumpir el tratamiento con estos medicamentos al menos dos semanas antes de empezar a tomar Duodopa solución para perfusión
- tiene un tumor en la glándula suprarrenal (feocromocitoma)
- tiene problemas hormonales como sobreproducción de cortisol (síndrome de Cushing) o sus hormonas tiroideas están muy elevadas (hipertiroidismo)
- en algún momento ha padecido cáncer de piel o presenta cualquier marca o lunar sospechoso en la piel que no haya sido controlado por su médico.
No use Duodopa solución para perfusión en cualquiera de los casos enumerados. Si no está seguro, consulte a su médico antes de usar este medicamento.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Duodopa solución para perfusión y durante su tratamiento si:
- ha tenido en algún momento un ataque al corazón, oclusión de los vasos sanguíneos o cualquier otra enfermedad del corazón, incluido un latido irregular (arritmia),
- tiene un problema pulmonar (como asma),
- ha sufrido en algún momento una enfermedad hormonal,
- ha tenido en algún momento depresión con pensamientos suicidas o cualquier otra enfermedad mental,
- tiene un trastorno ocular llamado “glaucoma de ángulo abierto”,
- ha tenido úlcera de estómago,
- ha tenido alguna vez ataques (convulsiones),
- tiene enfermedad renal o hepática,
- está siguiendo una dieta controlada en sodio (ver “Duodopa solución para perfusión contiene sodio”),
- sufre cualquier cambio en la piel del lugar de perfusión, como enrojecimiento, calor, hinchazón, dolor o cambio de color cuando se aplica presión en la zona,
- tiene debilidad progresiva, dolor, entumecimiento o pérdida de sensibilidad en los dedos o en los pies (polineuropatía). Su médico examinará estos signos y síntomas antes de que empiece el tratamiento con Duodopa solución para perfusión y periódicamente a partir de entonces. Informe a su médico si ya tiene neuropatía o un problema de salud asociado con neuropatía.
Si se encuentra en alguna de las situaciones mencionadas o si no está seguro de ello, consulte a su médico o farmacéutico antes de empezar el tratamiento con Duodopa solución para perfusión.
Informe a su médico si nota movimientos involuntarios e incontrolables en extremidades, espalda, cuello o barbilla (discinesias) o un aumento de la rigidez o enlentecimiento de los movimientos. Es posible que sea necesario un ajuste en la dosis diaria, o que el dispositivo pueda estar obstruido.
Síndrome neuroléptico maligno
No deje de usar Duodopa solución para perfusión a menos que su médico se lo indique. La interrupción del tratamiento o la bajada repentina de Duodopa solución para perfusión puede causar un trastorno grave llamado “síndrome neuroléptico maligno”. Los signos pueden incluir:
- latido cardiaco rápido, cambios en la tensión arterial y sudoración, seguidos de fiebre,
- respiración más rápida, rigidez muscular, disminución de la consciencia y coma,
- niveles más altos de una proteína en la sangre (una enzima llamada “creatina fosfoquinasa”). Su médico se encargará de medirla.
Trastornos de control de impulsos (cambios en su comportamiento)
Informe a su médico si usted, su familia o cuidador advierten que desarrolla impulsos o ansias de comportarse de manera inusual para usted, o usted no puede resistir el impulso, ímpetu o tentación de llevar a cabo ciertas actividades que puedan ser perjudiciales para usted o para otros. Estos comportamientos se llaman “trastornos de control de impulsos” e incluyen:
- adicción al juego,
- comer o gastar en exceso,
- incremento anormal del deseo sexual o aumento de los pensamientos o sentimientos sexuales.
Su médico puede necesitar revisar sus tratamientos. Hablará con usted sobre los métodos para controlar o reducir estos síntomas (ver sección 4 “Trastornos de control de impulsos - cambios en su comportamiento”).
Síndrome de disregulación de dopamina
Informe a su médico si usted, su familia o cuidador advierten que desarrolla síntomas similares a la adicción, que conducen a un deseo de grandes dosis de Duodopa solución para perfusión y otros medicamentos utilizados para tratar la enfermedad de Parkinson.
Infecciones en el lugar de perfusión
Informe a su médico si nota cualquier cambio en la piel del lugar de perfusión, como enrojecimiento, calor, hinchazón, dolor o cambio de color cuando se aplica presión en la zona. Debe seguir siempre técnicas asépticas (estériles) mientras esté utilizando este medicamento y cambiar regularmente el lugar de perfusión (al menos cada tres días) utilizando un equipo de perfusión nuevo. Asegúrese de que el nuevo lugar de perfusión esté al menos a 2,5 cm del lugar utilizado en los últimos 12 días. Es posible que tenga que cambiar el lugar de perfusión con más frecuencia que cada 3 días si nota cualquiera de los cambios mencionados anteriormente.
Duodopa solución para perfusión y cáncer
En el organismo, foscarbidopa (un componente de Duodopa solución para perfusión) se degrada transformándose en una sustancia llamada “hidrazina”. Es posible que la hidrazina pueda causar daño en el material genético lo que puede producir cáncer. Sin embargo, se desconoce si la cantidad de hidrazina que se produce con las dosis normales de Duodopa solución para perfusión puede causarlo.
Niños y adolescentes
Duodopa solución para perfusión no está recomendado en niños y adolescentes menores de 18 años de edad. Esto es debido a que el medicamento no se ha estudiado en este grupo de edad.
Otros medicamentos y Duodopa solución para perfusión
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento. Esto incluye los adquiridos sin receta médica, así como las plantas medicinales.
Consulte a su médico o farmacéutico antes de empezar a usar Duodopa solución para perfusión si está tomando otros medicamentos para:
- la tuberculosis, como isoniazida;
- la ansiedad, como benzodiacepinas;
- las náuseas o vómitos, como metoclopramida;
- la presión arterial alta, como antihipertensivos;
- los espasmos de los vasos sanguíneos, como papaverina;
- los ataques (convulsiones) o la epilepsia, como fenitoína;
- las enfermedades psiquiátricas, como antipsicóticos, que incluyen fenotiazinas, butirofenonas y risperidona;
- la enfermedad de Parkinson, como tolcapona, entacapona, opicapona y amantadina;
- la depresión, como antidepresivos tricíclicos que incluyen amoxapina y trimipramina.
Informe a su médico o farmacéutico si está tomando un inhibidor de la COMT (catecol-O-metil transferasa), ya que puede aumentar la cantidad de levodopa en su sangre. Su médico podría tener que ajustar la dosis de estos medicamentos.
Informe a su médico si está tomando medicamentos llamados fármacos simpaticomiméticos, como por ejemplo, salbutamol, fenilefrina, isoproterenol, dobutamina para tratar la presión arterial baja. Los medicamentos simpaticomiméticos y la levodopa pueden aumentar el riesgo de presión arterial alta (hipertensión) o latidos irregulares (arritmia).
Informe a su médico o farmacéutico si está tomando medicamentos que son eliminados por la acción de un enzima llamado ‘CYP1A2’. Por ejemplo:
- cafeína (ayuda a tener la mente alerta)
- melatonina (ayuda a regular el sueño)
- fluvoxamina, duloxetina (antidepresivos que mejoran el estado de ánimo)
- clozapina (para controlar la esquizofrenia)
- teofilina (ayuda con el asma)
Ciertos medicamentos (como selegilina) que pudiera tomar pueden bajar la presión arterial, lo que puede hacerle sentir mareado al levantarse de una silla o de la cama (hipotensión ortostática). Duodopa solución para perfusión puede empeorar esta sensación de mareo. Moverse lentamente cuando está tumbado o sentado puede hacerle sentir menos mareado.
No use Duodopa solución para perfusión si está tomando
- medicamentos para la depresión denominados inhibidores selectivos de la MAO-A e inhibidores no selectivos de la MAO, como moclobemida o fenelzina.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
No debe utilizarse este medicamento durante el embarazo ni en mujeres en edad fértil que no utilicen métodos anticonceptivos a menos que los beneficios para la madre superen los posibles riesgos para el feto.
Se desconoce si Duodopa solución para perfusión se excreta en la leche materna. Debe interrumpirse la lactancia durante el tratamiento con Duodopa solución para perfusión.
Conducción y uso de máquinas
No conduzca ni use herramientas o máquinas hasta que esté seguro de cómo le afecta Duodopa solución para perfusión.
- Duodopa solución para perfusión puede hacerle sentir una fuerte somnolencia, o puede que se descubra con frecuencia quedándose dormido de forma repentina (ataques de sueño).
- Duodopa solución para perfusión puede bajar la presión arterial, lo que puede hacerle sentir aturdido o mareado.
No conduzca o use cualquier herramienta o máquina hasta sentirse de nuevo completamente activo o no se sienta aturdido o mareado.
Duodopa solución para perfusión contiene sodio
Consulte a su médico o farmacéutico si necesita 9 ml o más de Duodopa solución para perfusión diarios por un periodo prolongado especialmente si le han recomendado una dieta baja en sal (sodio).
3. Cómo usar Duodopa solución para perfusión
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, enfermero o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cómo usar Duodopa solución para perfusión
- Antes de iniciar el tratamiento, usted, o su cuidador, recibirán formación sobre cómo manipular el producto y la bomba de perfusión.
- Duodopa solución para perfusión es una solución que se administra bajo la piel (denominada “perfusión subcutánea”), con más frecuencia en el abdomen, utilizando una bomba de perfusión. Debe evitar utilizar la bomba de perfusión en una zona circular de 5 cm alrededor del ombligo.
- Su médico o enfermero ajustarán los parámetros de la bomba para ajustar la dosis a sus necesidades.
- La bomba le administra de forma continua el medicamento durante 24 horas. Es posible que necesite recargar la bomba con una nueva jeringa dentro de un periodo de 24 horas para asegurarse de que administra suficiente medicamento en sangre para controlar sus síntomas.
Cuánto medicamento usar
- Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
- Su médico decidirá cuánta Duodopa solución para perfusión recibirá y por cuánto tiempo. Por lo general, se administrará una dosis continua de mantenimiento.
- Si lo necesita, puede recibir dosis extras (una opción disponible en su bomba) para tratar los síntomas “OFF” repentinos que puedan aparecer durante la perfusión continua; esto lo decidirá su médico.
- En caso de interrupciones superiores a 3 horas, también deberá autoadministrarse una dosis de carga (una opción disponible en su bomba) antes de reanudar la perfusión continua para restablecer rápidamente el control de los síntomas.
Consulte la sección 7, “Instrucciones de uso de Duodopa solución para perfusión con la bomba Vyafuser” antes de usar Duodopa solución para perfusión.
Si usa más Duodopa solución para perfusión del que debe
Si se ha administrado una dosis mayor de Duodopa solución para perfusión del que debería, interrumpa inmediatamente la perfusión y póngase en contacto con su médico, llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad administrada, o vaya a un hospital inmediatamente. Lleve el medicamento con usted. Pueden ocurrir los siguientes efectos:
- latidos del corazón inusuales rápidos, lentos o irregulares (arritmia).
- presión arterial baja (hipotensión).
Si olvidó usar o interrumpe el tratamiento con Duodopa solución para perfusión
Si olvidó usar Duodopa solución para perfusión, active su bomba, con su dosis habitual, lo antes posible.
La administración de Duodopa solución para perfusión puede interrumpirse durante periodos breves de tiempo, por ejemplo, cuando se esté duchando. Asegúrese de cambiar el equipo de perfusión (tubos y cánula) y cambie a un lugar de administración diferente si interrumpe la perfusión durante más de 1 hora. En caso de interrupciones superiores a 3 horas, también deberá autoadministrarse una dosis de carga para restablecer rápidamente el control de los síntomas. La opción de dosis de carga está disponible en su bomba según lo establecido por su médico o enfermero.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Deje de usar Duodopa solución para perfusión e informe a su médico inmediatamente si nota cualquiera de los siguientes efectos adversos graves. Podría necesitar tratamiento médico urgente:
- glaucoma de ángulo cerrado (dolor agudo en los ojos, dolor de cabeza, visión borrosa, náuseas y vómitos),
- hinchazón de la cara, la lengua o la garganta que puede dificultar tragar o respirar, o erupción cutánea de tipo urticaria. Pueden ser signos de una reacción alérgica grave (reacción anafiláctica). Frecuencia no conocida. No puede estimarse a partir de los datos disponibles.
Su médico decidirá si puede seguir utilizando Duodopa solución para perfusión.
Otros efectos adversos
Informe a su médico, farmacéutico o enfermero si nota cualquiera de los siguientes efectos adversos.
Muy frecuentes:pueden afectar a más de 1 de cada 10 pacientes
- infección en el lugar de perfusión (celulitis en el lugar de perfusión) (ver sección 2),
- ansiedad,
- ver, oír o percibir cosas que no existen (alucinaciones),
- depresión,
- reacciones en el lugar de perfusión (enrojecimiento, bulto, hinchazón y dolor),
- caídas,
- infecciones del tracto urinario.
Frecuentes:pueden afectar hasta a 1 de cada 10 pacientes
- reacciones en el lugar de perfusión (hematomas, descamación de la piel en capas finas piel, fuga del medicamento, sangrado, inflamación, irritación, bulto, protuberancia, picazón, sarpullido),
- abscesos en el lugar de perfusión,
- pérdida de apetito,
- confusión,
- falsas creencias (delirios),
- paranoia,
- pensamientos de quitarse la vida (pensamientos suicidas),
- problemas con la capacidad de pensar, aprender y recordar (trastorno cognitivo),
- movimientos involuntarios (discinesia),
- espasmos musculares incontrolables que afectan a los ojos, cabeza, cuello o cuerpo (distonía)
- dolor de cabeza,
- reducción del sentido del tacto, hormigueo o entumecimiento, sensación de quemazón o escozor en las manos, brazos, piernas o pies (hipoestesia, parestesia),
- debilidad progresiva o dolor o entumecimiento o pérdida de sensibilidad en los dedos o en los pies (polineuropatía),
- aparición inesperada o repentina de los síntomas de la enfermedad de Parkinson, esto se llama “fenómeno ON/OFF”,
- quedarse dormido de forma repentina (ataques de sueño), fuerte somnolencia, trastornos del sueño,
- presión arterial alta o baja,
- sensación de mareo,
- mareo, al levantarse o al cambiar de postura (hipotensión ortostática) debido a la bajada de presión arterial. Cambie siempre de postura lentamente, no se levante con rapidez,
- desmayo,
- dolor abdominal,
- estreñimiento,
- sequedad de boca,
- náuseas, diarrea o vómitos,
- incapacidad para controlar la orina (incontinencia),
- dificultad para orinar (retención de orina),
- falta de energía, sensación de debilidad (fatiga),
- hinchazón de las pantorrillas y las manos debido a un exceso de líquido (edema periférico)
- trastorno psicótico,
- niveles muy bajos de vitamina B6 en el cuerpo,
- niveles muy bajos de vitamina B12 en el cuerpo,
- aumento del número de aminoácidos, pequeñas moléculas que forman las proteínas del organismo,
- aumento de la cantidad de homocisteína en la sangre, que contribuye a la formación de proteínas en el organismo,
- dolor de garganta,
- aumento de peso,
- pérdida de peso,
- dificultad para dormir (insomnio),
- erupciones, picor, aumento de la sudoración,
- espasmos musculares,
- dificultad para respirar,
- sensación de malestar general,
- anemia,
- sueños anormales,
- agitación,
- tener el estómago hinchado (distensión abdominal), gases (flatulencia), indigestión (dispepsia)
- tener dolor,
- tener dolor de cuello,
- dificultad para tragar o cambio en el gusto (sabor amargo),
- latidos irregulares del corazón.
Trastornos de control de impulsos (cambios en su comportamiento)Son frecuentes, pueden afectar a 1 de cada 10 pacientes.
Algunas personas son incapaces de resistir el impulso de realizar una acción que podría ser perjudicial para ellos mismos o para otros. Esto puede incluir
- un fuerte impulso de jugar excesivamente a pesar de graves consecuencias personales o familiares,
- alteración o aumento del interés y el comportamiento sexual con gran preocupación para usted o para otros. Esto podría incluir un aumento del impulso sexual,
- compras o gasto incontrolado excesivo,
- comer en exceso (comer grandes cantidades de comida en un corto periodo de tiempo), o comer compulsivamente (comer más alimentos de lo normal y más de lo necesario para satisfacer el hambre).
Informe a su médico si usted, su familia o cuidador notan alguno de estos comportamientos. Su médico puede necesitar revisar su tratamiento. Discutirá con usted métodos para controlar o reducir estos síntomas.
Poco frecuentes:pueden afectar hasta a 1 de cada 100 pacientes
- deseo de grandes dosis de Duodopa solución para perfusión más allá de las requeridas para controlar los síntomas motores, lo que se conoce como síndrome de disregulación de dopamina,
- orina oscura,
- voz ronca, dolor de pecho,
- caída del pelo, piel enrojecida, habones,
- salivar más de lo normal,
- cambio en la forma de andar,
- intentar acabar o acabar con su propia vida, suicidio,
- número bajo de glóbulos blancos o cambios en los recuentos de células sanguíneas, lo que puede causar sangrado,
- estado de ánimo elevado (estado de ánimo eufórico), aumento del interés sexual, demencia, sensación de temor,
- problemas para controlar los movimientos y hacer movimientos bruscos que no puede controlar
- problemas para abrir los ojos, visión doble, visión borrosa, daño del nervio óptico (neuropatía óptica isquémica),
- latidos cardíacos irregulares que puede sentir (palpitaciones),
- confusión,
- pesadillas,
- hinchazón de una vena.
Raros:pueden afectar hasta a 1 de cada 1.000 pacientes
- rechinar de dientes
- erección dolorosa que no desaparece,
- marcas o lunares sospechosos en la piel que aparecen o empeoran, o tumores de la piel (melanoma maligno),
- saliva o sudor oscuro, sensación de quemazón en la lengua, hipo,
- pensamientos inusuales,
- respiración anormal.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Duodopa solución para perfusión
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar los viales en el embalaje exterior para evitar que se rompan.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del vial y en el envase. La fecha de caducidad es el último día del mes que se indica.
- No congelar.
- Conservar y transportar refrigerado (entre 2 ?C y 8 ?C).
- Los viales se pueden mantener a temperatura ambiente, hasta un máximo de 30 °C, durante un periodo único de un máximo de 28 días.
- En el envase se incluye un espacio para registrar el día en el que se sacó el medicamento de la nevera.
- Después de haber conservado el medicamento a temperatura ambiente, no debe devolverse a la nevera.
- Desechar el medicamento si no se utiliza durante el periodo de 28 días a temperatura ambiente.
- Se debe transferir a la jeringa el contenido completo del vial de una vez para su administración.
- No reutilizar un vial abierto; los viales son de un solo uso.
- Una vez abierto: utilizar inmediatamente. Utilizar Duodopa solución para perfusión en 24 horas una vez transferido del vial a la jeringa.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
- Desechar el vial después de transferir el medicamento a la jeringa.
- Desechar la jeringa y cualquier medicamento no utilizado que quede en la jeringa después de que el medicamento haya estado en la jeringa durante 24 horas.
6. Contenido del envase e información adicional
Composición de Duodopa solución para perfusión
- Los principios activos son foslevodopa y foscarbidopa. 1 ml contiene 240 mg de foslevodopa y 12 mg de foscarbidopa.
- 1 vial de 10 ml contiene 2.400 mg de foslevodopa y 120 mg de foscarbidopa.
- Los demás componentes son hidróxido sódico 10 N (para ajustar el pH), ácido clorhídrico concentrado (para ajustar el pH) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Duodopa solución para perfusión es una solución transparente a ligeramente opalescente, sin partículas, que se proporciona en un vial de vidrio transparente e incoloro con un tapón de goma gris y una tapa de plástico turquesa. El color de la solución puede variar de incolora a amarilla o marrón y puede tener tonalidad morada o roja. Están previstas variaciones de color y no afectan a la calidad del medicamento. La solución puede oscurecerse después de perforar el tapón del vial o mientras está en la jeringa.
Cada envase contiene 7 viales de 10 ml cada uno.
Los componentes de perfusión estériles de un solo uso (jeringa, equipo de perfusión y adaptador de viales) aptos para su uso se proporcionan por separado, consultando a su médico o farmacéutico. La bomba Vyafuser se suministra por separado.
Titular de la autorización de comercialización
AbbVie Spain, S.L.U.
Avenida de Burgos 91,
28050 Madrid, España
Responsables de la fabricación
AbbVie S.r.I
S.R. 148 Pontina Km 52 snc
04011 Campoverde di Aprilia (LT)
Italia
Fecha de la última revisión de este prospecto: diciembre 2023
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- Instrucciones de uso de Duodopa solución para perfusión con la bomba Vyafuser
Lea toda la siguiente sección antes de utilizar Duodopa solución para perfusión.
Información importante
Lea atentamente las siguientes instrucciones: estas instrucciones explican cómo preparar y usar Duodopa solución para perfusión.
- Duodopa solución para perfusión se administra mediante perfusión subcutánea con la ayuda de la bomba Vyafuser y sus componentes de perfusión (jeringa, equipo de perfusión y adaptador de vial).
- Puede obtener por separado los componentes de su equipo de perfusión, consultando a su médico o farmacéutico.
- También debe leer atentamente las instrucciones completas antes de usar Duodopa solución para perfusión
- instrucciones de uso del adaptador de vial
- instrucciones de uso del equipo de perfusión
- instrucciones de uso de la bomba Vyafuser para pacientes.
- Su médico o enfermero configurará la bomba para usted, para que siempre reciba la dosis correcta.
- Su médico o enfermero le dirá cómo administrarse el medicamento y cómo manejar la bomba antes de comenzar el tratamiento.
- En caso de duda, consulte a su médico o enfermero.
Como preparar su medicación
- No diluya la solución de Duodopa solución para perfusión ni llene la jeringa con cualquier otra solución.
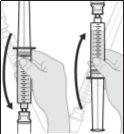
- El medicamento debe estar a temperatura ambiente antes de la perfusión. Si está refrigerado antes de su uso, saque el vial de la nevera y déjelo a temperatura ambiente alejado de la luz directa del sol durante 30 minutos. Si el medicamento está refrigerado, no lo caliente (ni en el vial ni en la jeringa) de ninguna manera que no sea dejarlo a temperatura ambiente. Por ejemplo, no lo caliente en el microondas ni en agua caliente.
- Preparación
Siga los siguientes pasos cada vez que necesite rellenar su bomba con Duodopa solución para perfusión.
- Lávese las manos con agua y jabón y séqueselas.
- Asegúrese de que la superficie lisa está limpia. Esto ayudará a evitar la contaminación cuando prepare la jeringa.
- Sobre una superficie lisa coloque
- Jeringa (en el interior de su envase)
- Vial de Duodopa solución para perfusión
- Adaptador de viales (en su envase). Se debe usar un nuevo adaptador de vial con cada nuevo vial de Duodopa solución para perfusión.
- Toallitas con alcohol (no proporcionadas con el medicamento)
- Compruebe la fecha de caducidad del vial, el adaptador de viales y la jeringa y que los envases no presentan daños.
- Noutilice el vial, el adaptador de viales ni la jeringa si sus respectivos envases estériles presentan daños.
- Noutilice la solución de Duodopa solución para perfusión, el adaptador de viales ni la jeringa si se ha pasado la fecha de caducidad.
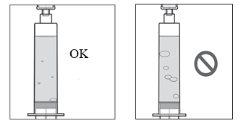
- Noutilice si la solución de Duodopa solución para perfusión está turbia o contiene escamas o partículas.
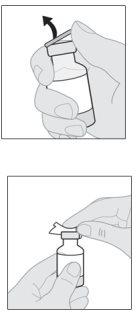
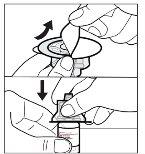
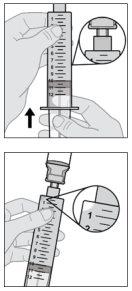
- Prepare el vial de solución
|
|
|
Puede que su adaptador de vial sea diferente al que se muestra en esta sección.
Para obtener información detallada, consulte las instrucciones de uso del adaptador del vial. |
|
|
|
|
|
|
|
|
|
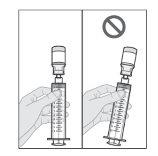
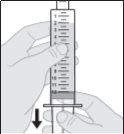
No agite ni golpee la jeringa para eliminar las burbujas de aire.
|
|
|
|
|
|
|
|
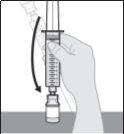
- Prepare la perfusión subcutánea de Duodopa solución para perfusión
|
|
|
- Elija y prepare el lugar de perfusión
Para más información, consulte las instrucciones de uso del equipo de perfusióny las instrucciones de uso de la bomba Vyafuser para pacientes. |
|
- Comience la perfusión subcutánea de Duodopa solución para perfusión
| ||||||||||||
|
- Después del uso
- Los viales de solución usados con el adaptador del vial aún conectado deben desecharse de acuerdo con las normativas locales o según las indicaciones de su médico, farmacéutico o enfermero.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DUODOPA 240 MG/ML + 12 MG/ML SOLUCION PARA PERFUSIONForma farmacéutica: COMPRIMIDO, 10 mg/100 mgPrincipio activo: levodopa and decarboxylase inhibitorFabricante: Fairmed Healthcare GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 12,5 mg/50 mgPrincipio activo: levodopa and decarboxylase inhibitorFabricante: Fairmed Healthcare GmbhRequiere recetaForma farmacéutica: COMPRIMIDO, 25 mg/100 mgPrincipio activo: levodopa and decarboxylase inhibitorFabricante: Fairmed Healthcare GmbhRequiere receta
Médicos online para DUODOPA 240 MG/ML + 12 MG/ML SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DUODOPA 240 MG/ML + 12 MG/ML SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes





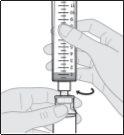
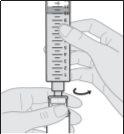
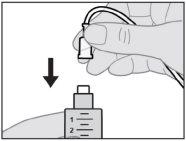 Acople el equipo de perfusión a la nueva jeringa.
Acople el equipo de perfusión a la nueva jeringa.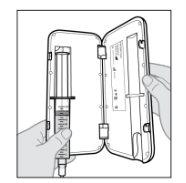
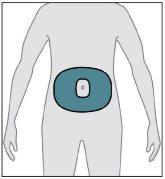 Del área que se muestra (en el abdomen), elija una zona separada al menos 5 cm del ombligo.
Del área que se muestra (en el abdomen), elija una zona separada al menos 5 cm del ombligo.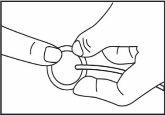 al cuerpo y el equipo de perfusión a la cánula.
al cuerpo y el equipo de perfusión a la cánula.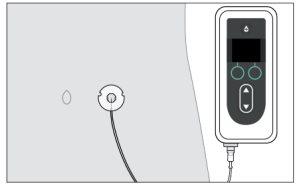 Inicie la bomba. Para más información, consulte las instrucciones de uso de la bomba Vyafuser.
Inicie la bomba. Para más información, consulte las instrucciones de uso de la bomba Vyafuser.


