BRONCHITOL 40 mg INHALATION POWDER, HARD CAPSULES

How to use BRONCHITOL 40 mg INHALATION POWDER, HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Bronchitol 40 mg powder for inhalation, hard capsules
Mannitol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Bronchitol and what is it used for
- What you need to know before you use Bronchitol
- How to use Bronchitol
- Possible side effects
- Storing Bronchitol
- Contents of the pack and other information
1. What is Bronchitol and what is it used for
What is Bronchitol
Bronchitol contains a medicine called mannitol, which is a mucolytic.
What is Bronchitol used for
Bronchitol is used in adults aged 18 years and older. In addition to using Bronchitol, the patient will continue to take their usual medication for cystic fibrosis.
How Bronchitol works
Bronchitol is inhaled into the lungs to improve cystic fibrosis, a genetic disease that affects the glands in the lungs, intestines, and pancreas that secrete fluids such as mucus and digestive juices.
Bronchitol helps to increase the amount of water present on the surface of the airways and in the mucus. This allows the lungs to clear the mucus more easily. It also helps to improve the condition of the lungs and breathing. As a result, a "productive cough" may occur, which also helps to clear the mucus from the lungs.
2. What you need to know before you use Bronchitol
Do not use Bronchitol
- if you are allergic (hypersensitive) to mannitol.
- if you are sensitive to mannitol. Before you start using Bronchitol, your doctor will check if your airways are too sensitive to mannitol. If you are too sensitive to mannitol, your airways will narrow and you may have difficulty breathing.
If any of the above applies to you (or if you are not sure), talk to your doctor or pharmacist before using this medicine.
Warnings and precautions
Talk to your doctor or pharmacist before using this medicine.
- if you have asthma;
- if you have ever coughed up blood or had blood in your sputum;
- if you have severe cystic fibrosis, especially if your lung function measured by forced expiratory volume in one second (FEV1) is usually below 30%,
Inhaling medicines can cause chest tightness and wheezing, in some cases immediately after taking the medicine. Your doctor will help you take your first dose of Bronchitol and check your lung function before, during, and after administration. Your doctor may ask you to use other medicines such as a bronchodilator before taking Bronchitol.
Inhaling medicines can also cause coughing, this may happen with Bronchitol. Talk to your doctor if the cough does not go away or worries you.
Children and adolescents
Bronchitol should not be used in children and adolescents under 18 years of age. This recommendation is due to the limited information available in this patient group.
Using Bronchitol with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
You can continue to take your cystic fibrosis medicines when using Bronchitol, these include inhaled antibiotics such as tobramycin and colistimethate sodium. If you are unsure, talk to your doctor or pharmacist before taking Bronchitol.
Pregnancy and breastfeeding
- If you are pregnant, think you may be pregnant, or plan to become pregnant, talk to your doctor before using this medicine. You should not take this medicine if you are pregnant.
- If you are breastfeeding or plan to breastfeed, talk to your doctor before using this medicine. It is not known if this medicine is excreted in breast milk.
Driving and using machines
Bronchitol is unlikely to affect your ability to drive or use tools or machines.
3. How to use Bronchitol
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are not sure, talk to your doctor or pharmacist.
Always use your bronchodilator before using Bronchitol.
Recommended dose
Adults aged 18 years and older
Initial dose
Before your doctor prescribes Bronchitol, they will help you take your first dose of Bronchitol and perform a lung function test at each step to check that you are not sensitive to mannitol. The first dose is taken in four steps:
Step 1 - 1 capsule (40 mg)
Step 2 - 2 capsules (80 mg)
Step 3 - 3 capsules (120 mg)
Step 4 - 4 capsules (160 mg)
At the end of the initial dose, you will have taken 10 capsules (400 mg), which is the normal dose.
Treatment dose (2-week packs)
- You should use Bronchitol every day.
- The normal dose is 10 capsules (400 mg) inhaled in the morning and 10 capsules inhaled at night.
- The evening dose should be taken at least 2 to 3 hours before bedtime.
- For best results, inhale the capsules one after the other, leaving as little time as possible between them.
Order for using this medicine
Use Bronchitol as part of your normal daily treatment routine. The suggested order is as follows, unless otherwise directed by your doctor:
- Use your bronchodilator
- Wait between 5 and 15 minutes
- Use Bronchitol before physiotherapy if it is part of your treatment routine.
- Dornase alfa (Pulmozyme) if it is part of your treatment routine
- Inhaled antibiotics if they are part of your treatment routine
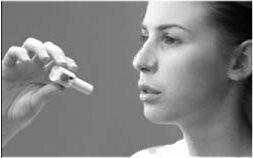
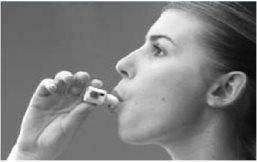
How to use Bronchitol
- Bronchitol is inhaled (breathed in) as a powder from a capsule using the inhaler provided in the pack. It should only be inhaled and not administered by any other route.
- The capsules should not be swallowed.
- Only inhale the powder contained in the capsules using the inhaler provided in the pack.
- Use a new inhaler each week.
- Place the ten capsules one by one into the inhaler.
- Inhale the contents of the capsule with the inhaler, taking one or two breaths.
For instructions on using the inhaler, see the end of the leaflet.
If you use more Bronchitol than you should
If you think you have taken too much of this medicine, talk to your doctor or pharmacist immediately. You may:
- feel like you cannot breathe;
- have wheezing;
- cough a lot.
Your doctor will give you oxygen and medicine to help you breathe.
If you forget to use Bronchitol
- If you forget a dose, use the inhaler as soon as you remember and continue as usual. However, if it is almost time for the next dose, skip the missed dose.
- Do not take a double dose to make up for forgotten doses.
If you stop using Bronchitol
If you stop using Bronchitol, your symptoms may get worse. Do not stop using Bronchitol without talking to your doctor first, even if you feel better. Your doctor will tell you how long you should use this medicine.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Bronchitol can cause side effects, although not everybody gets them.
Stop using Bronchitol and talk to a doctor immediately if you notice any of the following serious side effects:
- Difficulty breathing, which can be due to narrowing of the airways, worsening of asthma symptoms, or wheezing. These effects are common and may affect up to 1 in 10 people.
- Coughing up blood or blood in your sputum. This effect is common.
Tell your doctor immediately if you notice any of the following side effects:
- Severe cough. This effect is common.
- Worsening of symptoms. This effect is common.
Other side effects are:
Common (may affect up to 1 in 10 people)
- Cough
- Chest discomfort
- Headache
- Pain in the back of the mouth and throat and difficulty swallowing
- Vomiting, vomiting after coughing
Uncommon (may affect up to 1 in 100 people)
- Burning sensation or pain in the tongue
- Cystic fibrosis-related diabetes
- Chest and abdominal pain
- Change in voice
- Cold sweat
- Congestion
- Dehydration
- Decreased appetite
- Diarrhea
- Ear pain
- Feeling tired
- Feeling dizzy
- Nausea
- Feeling unwell
- Flu and fever
- Gas
- Heartburn
- Hernia pain
- Hyperventilation
- Itching, rash, acne
- Stiffness and joint pain
- Morbid thoughts
- Mouth sores
- Respiratory infection
- Runny nose
- Sputum infection
- Sore throat
- Difficulty sleeping
- Oral thrush (candidiasis)
- Loss of bladder control
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V*. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Bronchitol
Keep out of the sight and reach of children.
Do not use Bronchitol after the expiry date which is stated on the carton and blister after 'EXP'. The expiry date is the last day of the month stated.
Store below 30°C.
Store in the original package to protect from moisture.
Once removed from the blister, the capsule should be used immediately.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Bronchitol Composition
The active ingredient is mannitol. Each capsule contains 40 mg of mannitol. The average inhaled dose per capsule is 32.2 milligrams of mannitol.
Product Appearance and Package Contents
Bronchitol is a powder for inhalation in hard capsules. Bronchitol 40 mg powder for inhalation, hard capsules, is a white or almost white powder in hard, transparent, and colorless capsules with the inscription «PXS 40 mg». The powder is inhaled into the lungs with the inhaler provided in the package.
Each initial dose package of Bronchitol contains 1 blister pack with 10 capsules and 1 inhaler. The initial dose package is used for the initial dose evaluation with your doctor.
Each 2-week treatment package of Bronchitol contains 28 blister packs with 10 capsules each (280 capsules in total) and 2 inhalers. The 2-week treatment package is for therapeutic use.
Marketing Authorization Holder and Manufacturer
Pharmaxis Europe Limited, 108 Q House, Furze Road, Sandyford, Dublin 18, D18AY29, Ireland
Manufacturer
MIAS Pharma Limited, Suite 1, Stafford House, Strand Road, Portmarnock, Co. Dublin, D13WC83, Ireland or Arvato Supply Chain Solutions SE, Gottlieb-Daimler Straβe 1, 33428 Harsewinkel, North Rhine-Westphalia, Germany.
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 | Lithuania Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Greece Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 | Luxembourg Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Czech Republic 4 Life Pharma CZ, s.r.o. Tel: +420 244 403 003 | Hungary Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Denmark Chiesi Pharma AB Tel: + 46 8 753 35 20 | Malta Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Germany Chiesi GmbH Tel: +49 (0) 40 897 240 | Netherlands Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Estonia Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 | Norway Chiesi Pharma AB Tel: + 46 8 753 35 20 |
Greece Chiesi Hellas A.E.B.E. Tel: + 30.210.617.97.63 | Austria Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Spain Chiesi España, S.A.U. Tel: +34 93 494 8000 | Poland IMED Poland Sp. z o. o. Tel: +48 22 663 43 10 |
France Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 | Portugal Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Croatia Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 | Romania Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Ireland Cheisi Limited Tel: + 44 (0)161 488 5555 | Slovenia Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 |
Iceland Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 | Slovakia 4 Life Pharma SK, s.r.o. Tel: + 420 244 403 003 |
Italy Chiesi Farmaceutici S.p.A Tel: +39 0521 2791 | Finland Chiesi Pharma AB Tel: + 46 8 753 35 20 |
Cyprus Chiesi Hellas A.E.B.E. Tel: + 30.210.617.97.63 | Sweden Chiesi Pharma AB Tel: + 46 8 753 35 20 |
Latvia Pharmaxis Europe Limited Tel: + 353 (0) 1431 9816 | United Kingdom Cheisi Limited Tel: + 44 (0)161 488 5555 |
Date of the Last Revision of this Prospectus: MM/YYYY.
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites about rare diseases and orphan medicines.
------------------------------------------------------------------------------------------------------------------>
How to Use the Inhaler
The following diagram explains how the inhaler works. Bronchitol capsules can only be used with the inhaler provided in the package.
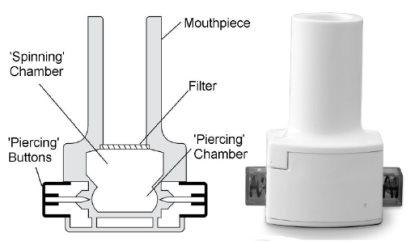
Spinning Chamber: Spiral Chamber
Mouthpiece: Mouthpiece
Filter: Filter
Piercing Buttons: Piercing Buttons
Piercing Chamber: Piercing Chamber
Inhaler
The following steps explain how to use the inhaler. If you want more information on how to care for it, go to the end of the instructions.
|
|
|
|
|
|
|
|
| |
|
|
| |
|
|
|
|
| |
|
Additional Information on Inhaler Care
- Keep the inhaler dry and make sure your hands are dry before using it.
- Never breathe or cough into the inhaler.
- Never disassemble it.
- Never place a capsule directly into the inhaler mouthpiece.
- Never leave a capsule inside the inhaler chamber.
- Use a new inhaler every week.
- If the inhaler breaks, use the second inhaler and talk to your doctor.
Cleaning the Inhaler -Normally, the inhaler will deliver the correct dose of medication for 7 days without needing cleaning. If you need to clean it, follow these steps:
- Make sure the inhaler is empty.
- Wash it with hot water with the mouthpiece open.
- Shake it to remove excess water until large drops stop falling from it.
- Let the inhaler air dry: lay it on its side with the mouthpiece open.
- Let it dry completely, which may take up to 24 hours. While it is drying, use the other inhaler.
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRONCHITOL 40 mg INHALATION POWDER, HARD CAPSULESDosage form: EFFERVESCENT TABLET, 600 mgActive substance: acetylcysteineManufacturer: Kern Pharma S.L.Prescription not requiredDosage form: EFFERVESCENT TABLET, 600 mgActive substance: acetylcysteineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: EFFERVESCENT TABLET, 200 mgActive substance: acetylcysteineManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for BRONCHITOL 40 mg INHALATION POWDER, HARD CAPSULES
Discuss questions about BRONCHITOL 40 mg INHALATION POWDER, HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions