SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO

Pergunte a um médico sobre a prescrição de SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO

Como usar SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO
Introdução
Prospecto: informação para o utilizador
Spiriva Respimat 2,5 microgramas, solução para inalação
tiotrópio
Leia todo o prospecto atentamente antes de começar a tomar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto:
- O que é Spiriva Respimat e para que é utilizado
- O que precisa saber antes de começar a tomar Spiriva Respimat
- Como tomar Spiriva Respimat
- Possíveis efeitos adversos
- Conservação de Spiriva Respimat
- Conteúdo do envase e informação adicional
1. O que é Spiriva Respimat e para que é utilizado
Spiriva Respimat ajuda os pacientes com doença pulmonar obstrutiva crónica (DPOC) ou asma a respirar mais facilmente. A DPOC é uma doença pulmonar a longo prazo que causa dificuldade para respirar e tosse. O termo DPOC está associado a bronquite crónica e enfisema. A asma é uma doença a longo prazo que se caracteriza por inflamação e estreitamento das vias respiratórias.
Como a DPOC e a asma são doenças a longo prazo, deve tomar Spiriva Respimat todos os dias e não apenas quando tiver problemas respiratórios ou outros sintomas. Quando é utilizado para tratar a asma, deve tomar Spiriva Respimat além dos chamados corticosteroides inhalados e agonistas beta2 de ação prolongada.
Spiriva Respimat é um broncodilatador de ação prolongada que ajuda a abrir as suas vias respiratórias e permite tomar e expulsar ar dos pulmões mais facilmente. O uso regular de Spiriva Respimat pode ajudá-lo também quando tem dificuldade contínua para respirar devida à sua doença, e ajudá-lo a minimizar os efeitos da doença na sua vida diária. O uso diário de Spiriva Respimat também o ajudará a prevenir qualquer piora repentina e a curto prazo dos sintomas da sua DPOC que podem durar vários dias.
Para uma dosagem correta de Spiriva Respimat, ver seção 3. Como tomar Spiriva Respimat e as instruções sobre como usar o inhalador Respimat recarregável incluídas no final do prospecto.
2. O que precisa saber antes de começar a tomar Spiriva Respimat
Não tome Spiriva Respimat
- se é alérgico (hipersensível) ao tiotrópio ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6).
- se é alérgico (hipersensível) à atropina ou substâncias relacionadas, por exemplo, ipratrópio ou oxitrópio.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a tomar Spiriva Respimat.
Consulte o seu médico se sofre de glaucoma de ângulo estreito, problemas de próstata ou tem dificuldades para urinar.
Se tiver problemas renais, por favor, consulte o seu médico.
Quando usar Spiriva Respimat, tenha cuidado para que não entre produto nos seus olhos. Isso pode provocar dor ou desconforto ocular, visão borrosa, halos visuais ou imagens coloridas associadas a vermelhidão dos olhos (ou seja, glaucoma de ângulo estreito). Os sintomas oculares podem estar acompanhados de dor de cabeça, náuseas ou vómitos. Lave os olhos com água morna, interrompa o uso de brometo de tiotrópio e consulte imediatamente o seu médico para mais informações.
Se a sua respiração piorar ou aparecer erupção, inflamação ou picazão logo após utilizar o seu inhalador, pare de utilizá-lo e entre em contato imediatamente com o seu médico.
A secura da boca que se observou com o tratamento com anticolinérgicos pode, a longo prazo, associar-se a cáries dentárias. Portanto, lembre-se de cuidar da sua higiene bucal.
Spiriva Respimat está indicado para o tratamento de manutenção da sua doença pulmonar obstrutiva crónica ou asma. Não tome este medicamento para tratar um episódio repentino de falta de respiração ou pitos (sibilâncias). O seu médico deve ter-lhe dado outro inhalador (“medicação de resgate”) para isso. Siga as instruções que o seu médico lhe deu.
Se lhe foi prescrito Spiriva Respimat para a asma, deve tomá-lo adicionado ao seu tratamento que inclui corticosteroides inhalados e agonistas beta2 de ação prolongada. Continue tomando corticosteroides inhalados conforme prescrito pelo seu médico, mesmo que se sinta melhor.
Em caso de ter sofrido um infarto do miocárdio nos últimos 6 meses ou batimentos cardíacos irregulares instáveis, ou que tenham colocado em perigo a sua vida, ou falha cardíaca grave no último ano, informe o seu médico. Isso é importante para decidir se Spiriva é o medicamento adequado para si.
Não tome Spiriva Respimat mais de uma vez ao dia.
Entre em contato também com o seu médico se sentir que a sua respiração piorou.
Se tiver fibrose cística, informe o seu médico, pois Spiriva Respimat pode fazer com que os seus sintomas de fibrose cística piorem.
Crianças e adolescentes
Spiriva Respimat não é recomendado para crianças menores de 6 anos.
Outros medicamentos e Spiriva Respimat
Informa o seu médico ou farmacêutico se está tomando, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Especially informe o seu médico ou farmacêutico se está tomando ou tomou medicamentos anticolinérgicos, como por exemplo, ipratrópio ou oxitrópio.
Não foi relatado reações adversas de interação quando Spiriva Respimat foi tomado com outros medicamentos utilizados habitualmente para tratar a DPOC e a asma, como os inhaladores de resgate (p. ex., salbutamol), metilxantinas (p. ex., teofilina), antihistamínicos, mucolíticos (p. ex., ambroxol), modificadores de leucotrienos (p. ex., montelukast), tratamento com anti-IgE (p. ex., omalizumab) e/ou esteroides orais ou inhalados (p. ex., budesonida, prednisolona).
Gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico antes de utilizar este medicamento.
Deve evitar tomar este medicamento a menos que o seu médico o recomende especificamente.
Condução e uso de máquinas
Não foram realizados estudos sobre os efeitos na capacidade para conduzir e utilizar máquinas. A ocorrência de tontura ou visão borrosa pode influir na capacidade para conduzir e utilizar máquinas.
Spiriva Respimat contém Cloruro de benzalconio
Este medicamento contém 0,0011 mg de cloruro de benzalconio em cada pulsação.
O cloruro de benzalconio pode provocar sibilâncias e dificuldades respiratórias (broncoespasmo), especialmente em pacientes com asma.
3. Como tomar Spiriva Respimat
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, pergunte ao seu médico ou farmacêutico.
Spiriva Respimat deve ser utilizado apenas por inalação.
A dose recomendada para pacientes maiores de 6 anos é:
Spiriva Respimat é eficaz durante 24 horas, pelo que necessitará tomar Spiriva Respimat apenas UMA VEZ AO DIA, se possível à mesma hora do dia .Cada vez que o utilize, realize DUAS PULSAÇÕES.
Como a DPOC e a asma são doenças a longo prazo, tome Spiriva Respimat todos os dias e não apenas quando tiver problemas respiratórios. Não tome mais doses do que a recomendada.
Certifique-se de que sabe como utilizar corretamente o seu inhalador Respimat recarregável. As instruções de uso do inhalador Respimat recarregável encontram-se no final deste prospecto, ver “Instruções sobre como usar o inhalador Respimat recarregável”.
Uso em crianças e adolescentes
Spiriva Respimat não é recomendado para uso em crianças menores de 6 anos devido à ausência de dados sobre segurança e eficácia.
Se tomar mais Spiriva Respimat do que devia
Se tomar mais de duas pulsações de Spiriva Respimat em um dia, consulte o seu médico imediatamente. Pode ter um maior risco de sofrer um efeito adverso, como secura da boca, constipação, dificuldade para urinar, aumento do ritmo cardíaco ou visão borrosa.
Em caso de sobredosagem ou ingestão acidental, consulte imediatamente o seu médico, farmacêutico ou ligue para o Serviço de Informação Toxicológica, telefone 91 562 04 20, indicando o medicamento e a quantidade administrada.
Se esquecer de tomar Spiriva Respimat
Se esquecer de tomar uma dose (DUAS PULSAÇÕES AO DIA), tome-a assim que se lembrar, mas não tome duas doses ao mesmo tempo ou no mesmo dia. Em seguida, tome a sua próxima dose como sempre.
Se interromper o tratamento com Spiriva Respimat
Antes de interromper o tratamento com Spiriva Respimat, deve falar com o seu médico ou farmacêutico. Se interromper o tratamento com Spiriva Respimat, os sinais e sintomas da sua DPOC e asma podem piorar.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
A avaliação dos efeitos adversos está baseada nas seguintes frequências:
Frequentes: podem afetar até 1 em cada 10 pessoas Pouco frequentes: podem afetar até 1 em cada 100 pessoas Raras: podem afetar até 1 em cada 1.000 pessoas Frequência não conhecida: a frequência não pode ser estimada a partir dos dados disponíveis |
Os efeitos adversos listados a seguir estão baseados na experiência de pessoas que tomaram este medicamento e estão listados de acordo com a frequência como frequentes, pouco frequentes, raros ou não conhecidos.
Efeitos Adversos | Frequência DPOC | Frequência Asma |
Secura da boca | Frequente | Pouco frequente |
Tontura | Pouco frequente | Pouco frequente |
Dor de cabeça (cefaleia) | Pouco frequente | Pouco frequente |
Dificuldade para dormir (insónia) | Rara | Pouco frequente |
Latidos cardíacos irregulares (fibrilação auricular, taquicardia supraventricular) | Rara | Não conhecida |
Notar o latido do coração (palpitações) | Rara | Pouco frequente |
Latidos cardíacos mais rápidos (taquicardia) | Rara | Não conhecida |
Tosse | Pouco frequente | Pouco frequente |
Sangramento do nariz (epistaxe) | Rara | Rara |
Inflamação da garganta (faringite) | Pouco frequente | Pouco frequente |
Ronquera (disfonia) | Pouco frequente | Pouco frequente |
Opresão no peito, associada a tosse, pitos ou falta de respiração imediatamente após a inalação (broncoespasmo) | Rara | Pouco frequente |
Constipação | Pouco frequente | Rara |
Infecções por fungos na boca e garganta (candidíase orofaríngea) | Pouco frequente | Pouco frequente |
Dificuldade para engolir (disfagia) | Rara | Não conhecida |
Erupção | Pouco frequente | Pouco frequente |
Picazão (prurido) | Pouco frequente | Rara |
Dificuldade para urinar (retenção de urina) | Pouco frequente | Não conhecida |
Dor ao urinar (disúria) | Pouco frequente | Não conhecida |
Halos visuais (luzes difusas) ou imagens coloridas associadas a vermelhidão dos olhos (glaucoma) | Rara | Não conhecida |
Aumento da pressão ocular | Rara | Não conhecida |
Visão borrosa | Rara | Não conhecida |
Inflamação da laringe (laringite) | Rara | Não conhecida |
Ardor de estômago (refluxo gastroesofágico) | Rara | Não conhecida |
Cáries dentárias | Rara | Não conhecida |
Inflamação das gengivas (gengivite) | Rara | Rara |
Inflamação da língua (glossite) | Rara | Não conhecida |
Inflamação da boca (estomatite) | Não conhecida | Rara |
Reação alérgica grave que pode causar inchaço da boca e face ou garganta (edema angioneurótico) | Rara | Rara |
Erupção irritante (urticária) | Rara | Rara |
Infecção ou ulceração da pele | Rara | Não conhecida |
Secura da pele | Rara | Não conhecida |
Hipersensibilidade, incluindo reações imediatas | Não conhecida | Rara |
Infecção do trato urinário | Rara | Rara |
Perda de água corporal (desidratação) | Não conhecida | Não conhecida |
Sinusite | Não conhecida | Não conhecida |
Obstrução do intestino ou ausência de movimento do intestino grosso (obstrução intestinal, incluindo íleo paralítico) | Não conhecida | Não conhecida |
Náuseas | Não conhecida | Não conhecida |
Reação alérgica grave (reação anafilática) | Não conhecida | Não conhecida |
Inflamação das articulações | Não conhecida | Não conhecida |
Após a administração de Spiriva Respimat, podem ocorrer, de forma individual ou como parte de uma reação alérgica grave (reação anafilática), reações alérgicas imediatas, tais como erupção, erupção irritante (urticária), inchaço da boca e da face ou garganta (edema angioneurótico) ou dificuldade repentina para respirar, ou outras reações de hipersensibilidade (tais como diminuição repentina da pressão arterial ou tontura).
Além disso, como com todos os medicamentos inhalados, alguns pacientes podem experimentar opressão inesperada no peito, tosse, pitos ou falta de respiração, imediatamente após a inalação (broncoespasmo).
Se ocorrer qualquer uma dessas reações, por favor, consulte imediatamente o seu médico.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Español de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Spiriva Respimat
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no estojo e na etiqueta do cartucho. A data de validade é o último dia do mês que se indica.
Não congele.
Período de validade em uso:
Troque o cartucho como máximo três meses após a sua inserção.
Não use o inhalador Respimat recarregável durante mais de um ano.
Uso recomendado: 6 cartuchos por inhalador.
Nota: o funcionamento do inhalador RESPIMAT recarregável foi demonstrado em testes para 540 pulsações (correspondente a 9 cartuchos).
Os medicamentos não devem ser jogados nos deságues nem na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da sua farmácia habitual. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Spiriva Respimat
O princípio ativo é tiotrópio. A dose liberada é de 2,5 microgramas de tiotrópio por pulsação (2 pulsações são uma dose) e equivale a 3,124 microgramas de brometo de tiotrópio monohidrato.
A dose liberada é a dose disponível para o paciente após passar pela boquilla.
Os demais componentes são cloreto de benzalconio, edetato dissódico, água purificada e ácido clorídrico 3,6 % para ajustar o pH.
Aspecto do produto e conteúdo do envase
Spiriva Respimat 2,5 microgramas compõe-se de um cartucho com a solução para inalação e um inhalador Respimat. O cartucho deve ser inserido no inhalador antes da primeira utilização.
Envase individual: 1 inhalador Respimat recarregável e 1 cartucho que proporciona 60 pulsações (30 doses).
Envase triplo: 1 inhalador Respimat recarregável e 3 cartuchos que proporcionam 60 pulsações (30 doses) cada um.
Envase individual de recambio: 1 cartucho que proporciona 60 pulsações (30 doses).
Envase triplo de recambio: 3 cartuchos que proporcionam 60 pulsações (30 doses) cada um.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
O título da autorização de comercialização de Spiriva Respimat é:
Boehringer Ingelheim International GmbH
Binger Strasse 173
55216 Ingelheim am Rhein
Alemanha
O responsável pela fabricação de Spiriva Respimat é:
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
55216 Ingelheim am Rhein
Alemanha
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
Espanha
Boehringer Ingelheim France
100-104 Avenue de France
75013 Paris
França
Representante local:
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
Espanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu e no Reino Unido (Irlanda do Norte) com os seguintes nomes:
Áustria, Liechtenstein, Bélgica, Luxemburgo, Chipre, República Checa, Dinamarca, Estónia, Finlândia, França, Alemanha, Grécia, Hungria, Islândia, Irlanda, Malta, Reino Unido (Irlanda do Norte), Itália, Letónia, Lituânia, Holanda, Noruega, Polónia, Portugal, Roménia, Eslováquia, Eslovénia, Espanha, Suécia: Spiriva Respimat
Bulgária: ??????? ????????
Data da última revisão deste prospecto:Dezembro 2024
A informação detalhada e atualizada sobre este medicamento está disponível no site da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es.
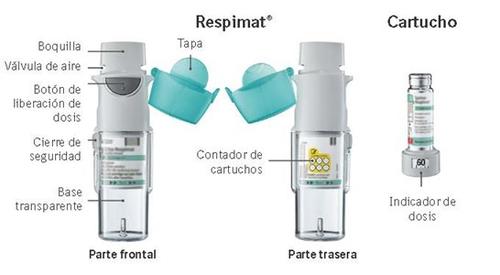
Instruções sobre como usar o inhalador Respimat recarregável
Respimat é um dispositivo inhalador que gera um aerossol para inalação. Respimat é exclusivamente para você. Um cartucho proporciona várias doses. O inhalador Respimat recarregável permite substituir o cartucho e pode ser usado até com 6 cartuchos.
As crianças devem usar Spiriva Respimat com a ajuda de um adulto.
Leia estas instruções antes de começar a usar Spiriva Respimat.
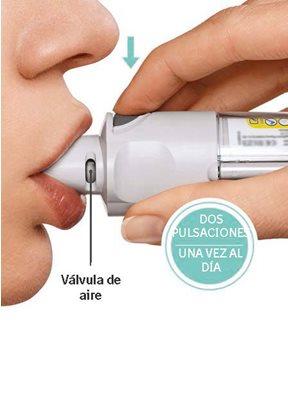
 Necessitará usar este inhalador UMA VEZ AO DIA. Cada vez que o usar, realize DUAS PULSAÇÕES.
Necessitará usar este inhalador UMA VEZ AO DIA. Cada vez que o usar, realize DUAS PULSAÇÕES.
- Se Spiriva Respimat não foi utilizado durante mais de 7 dias, realize uma pulsação em direção ao chão.
- Se Spiriva Respimat não foi utilizado durante mais de 21 dias, repita os passos do 4 ao 6, descritos em “Preparação para o uso” até que observe uma nuvem. Então repita os passos do 4 ao 6 três vezes mais.
Como manter seu inhalador Respimat recarregável
Limpe a boquilla, incluindo a parte metálica que se encontra dentro da mesma, apenas com um pano úmido ou um lenço, pelo menos uma vez por semana.
Qualquer pequena descoloração da boquilla não afeta o funcionamento do seu inhalador Respimat recarregável.
Se necessário, limpe a parte exterior do seu inhalador Respimat recarregável com um pano úmido.
Quando cambiar o inhalador
Quando tiver usado 6 cartuchos com o mesmo inhalador, obtenha um novo envase de Spiriva Respimat contendo um inhalador. Não use o inhalador Respimat recarregável durante mais de um ano, após ter inserido o primeiro cartucho.

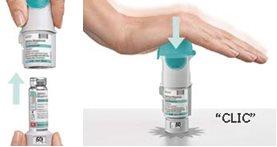
Preparação para o uso
|
|
|
|
|
|
|
|
|
|
Agora seu inhalador está pronto para ser utilizado e liberará 60 pulsações (30 doses). |
|
Uso diário
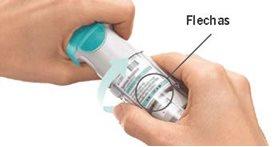
GIRAR
|
|
ABRIR
|
|
PULSAR
|
|
Quando cambiar o cartucho de Spiriva Respimat
O indicador de dose mostra quantas pulsações restam no cartucho.
 Restam 60 pulsações.
Restam 60 pulsações.
 Restam menos de 10 pulsações. Obtenha um novo cartucho.
Restam menos de 10 pulsações. Obtenha um novo cartucho.
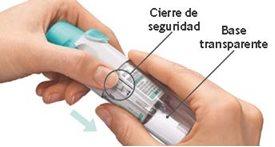
 Seu cartucho se esgotou. Gire a base transparente para afrouxá-la. Seu inhalador agora está bloqueado. Retire o cartucho do inhalador. Insira um novo cartucho até que faça um clique (consulte o passo 2). O novo cartucho sobressairá mais que o primeiro cartucho (continue com o passo 3). Lembre-se de recolocar a base transparente para desbloquear o inhalador.
Seu cartucho se esgotou. Gire a base transparente para afrouxá-la. Seu inhalador agora está bloqueado. Retire o cartucho do inhalador. Insira um novo cartucho até que faça um clique (consulte o passo 2). O novo cartucho sobressairá mais que o primeiro cartucho (continue com o passo 3). Lembre-se de recolocar a base transparente para desbloquear o inhalador.
Respostas a perguntas frequentes
É difícil inserir o cartucho o suficiente.
Já girou acidentalmente a base transparente antes de inserir o cartucho?Abra a tampa, pulse o botão liberador de dose e então insira o cartucho.
Está mudando o cartucho?O novo cartucho sobressairá mais que o primeiro cartucho. Insira o cartucho até que faça um clique, depois coloque a base transparente.
Não consigo pulsar o botão liberador de dose.
Já recolocou a base transparente?Se não o fez, recoloque a base transparente para desbloquear o inhalador. O inhalador Respimat recarregável só funciona com a base transparente colocada.
Já girou a base transparente?Se não o fez, gire a base transparente com um movimento contínuo até que faça um clique (meia-volta).O indicador de dose do seu cartucho mostra uma seta branca sobre um fundo vermelho?Seu cartucho se esgotou. Insira um novo cartucho.
É difícil retirar o cartucho uma vez que se esgotou.
Puxe e gire o cartucho ao mesmo tempo.
Não consigo girar ou recolocar a base transparente.
A base transparente está frouxa e o indicador de dose do seu cartucho mostra uma seta branca sobre um fundo vermelho?Seu cartucho se esgotou. Insira um novo cartucho.
Já girou a base transparente?Se já girou a base transparente, siga os passos “ABRIR” e “PULSAR” descritos em “Uso diário” para usar seu medicamento.
Meu Spiriva Respimat se esgotou muito cedo.
Usou SpirivaRespimat como indicado (duas pulsações/uma vez ao dia)?Cada cartucho durará 30 dias se forem realizadas duas pulsações uma vez ao dia.
Usou SpirivaRespimat comfrequência para testar se Spiriva Respimat funciona?Uma vez que tenha preparado Spiriva Respimat para uso, não é necessário testar seu funcionamento pulverizando a solução se for usado diariamente.
Retirou e recolocou a base transparente várias vezes?Não retire a base transparente antes que o cartucho se esgote. Cada vez que você retira a base transparente sem mudar o cartucho, o contador de dose registra uma pulsação e as doses restantes são reduzidas.
Meu Spiriva Respimat não pulveriza.Inseriu um cartucho?Se não, insira um cartucho. Uma vez que seu Spiriva Respimat está montado, não retire a base transparente ou o cartucho até que se esgote o cartucho.
Repetiu os passosGIRAR, ABRIR, PULSARmenos de três vezes após inserir o cartucho?Repita os passos GIRAR, ABRIR, PULSAR três vezes após inserir o cartucho, como descrito nos passos 4 a 6 em “Preparação para o uso”.
O indicador de dose do seu cartucho mostra uma seta branca sobre um fundo vermelho?Seu cartucho se esgotou. Insira um novo cartucho.
Meu Spiriva Respimat pulveriza automaticamente.
A tampa estava aberta quando girou a base transparente?Feche a tampa, então gire a base transparente.
Pulsou o botão de liberação de dose enquanto girava a base transparente?Feche a tampa, de modo que o botão de liberação de dose fique coberto, então gire a base transparente.
Parou de girar a base transparente antes que fizesse um clique?Gire a base transparente com um movimento contínuo até que faça um clique (meia-volta). O contador de dose contará cada giro incompleto e o número de doses restantes será reduzido.
A tampa estava aberta quando mudou o cartucho?Feche a tampa, então mude o cartucho.
Outras fontes de informação
Pode acessar informações detalhadas e atualizadas sobre como administrar este medicamento escaneando com seu telefone móvel (smartphone) o código QR incluído na seção “Instruções sobre como usar o inhalador Respimat recarregável” deste prospecto e no cartonagem. Também pode acessar esta informação no seguinte endereço de internet: https://cima.aemps.es/info/69589.


Quanto custa o SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO em Espanha em 2025?
O preço médio do SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO em dezembro de 2025 é de cerca de 39.25 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia39.25 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃOForma farmacêutica: INALAÇÃO PULMONAR, 10 MICROGRAMASSubstância ativa: tiotropium bromideFabricante: Teva Pharma S.L.U.Requer receita médicaForma farmacêutica: INALAÇÃO PULMONAR, 10 microgramasSubstância ativa: tiotropium bromideFabricante: Teva Pharma S.L.U.Requer receita médicaForma farmacêutica: INALAÇÃO PULMONAR, 18 microgramasSubstância ativa: tiotropium bromideFabricante: Boehringer Ingelheim International GmbhRequer receita médica
Alternativas a SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO em Polónia
Alternativa a SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO em Ukraine
Médicos online para SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de SPIRIVA RESPIMAT 2,5 microgramas SOLUÇÃO PARA INALAÇÃO – sujeita a avaliação médica e regras locais.