PERGOVERIS 150 UI/75 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL


Como usar PERGOVERIS 150 UI/75 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Introdução
Prospecto: informação para o utilizador
Pergoveris 150 UI/75 UI pó e diluente para solução injectável
folitropina alfa/lutropina alfa
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que o reler.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Pergoveris e para que é utilizado
- O que precisa saber antes de começar a usar Pergoveris
- Como usar Pergoveris
- Efeitos adversos possíveis
- Conservação de Pergoveris
- Conteúdo do envase e informações adicionais
1. O que é Pergoveris e para que é utilizado
O que é Pergoveris
Pergoveris contém dois princípios ativos diferentes denominados “folitropina alfa” e “lutropina alfa”. Ambos pertencem à família de hormonas chamadas “gonadotropinas”, que estão implicadas na reprodução e na fertilidade.
Para que é utilizado Pergoveris
Este medicamento é utilizado para estimular o desenvolvimento dos folículos (cada um contendo um óvulo) nos ovários com o fim de ajudá-la a engravidar. Está destinado ao uso em mulheres adultas (18 anos de idade ou mais) com níveis baixos (déficit grave) de “hormona foliculoestimulante” (FSH) e “hormona luteinizante” (LH). Normalmente, estas mulheres são inférteis.
Como actua Pergoveris
Os princípios ativos de Pergoveris são cópias das hormonas naturais FSH e LH. No corpo:
- a FSH estimula a produção de óvulos
- a LH estimula a libertação dos óvulos.
Ao substituir as hormonas ausentes, Pergoveris permite às mulheres com níveis baixos de FSH e LH desenvolver um folículo, a partir do qual se libertará um óvulo, após uma injeção da hormona “gonadotropina coriónica humana (hCG)”. Isso ajuda as mulheres a engravidar.
2. O que precisa saber antes de começar a usar Pergoveris
Antes de iniciar o tratamento deve ser avaliada a sua fertilidade e a do seu parceiro por parte de um médico experiente no tratamento dos distúrbios da fertilidade.
Não use Pergoveris:
- se é alérgico à hormona foliculoestimulante (FSH), à hormona luteinizante (LH) ou a algum dos outros componentes deste medicamento (incluídos na secção 6).
- se tem um tumor cerebral (no hipotálamo ou na hipófise).
- se tem ovários grandes ou bolsas de líquido no interior dos ovários (quistes ováricos) de origem desconhecida.
- se tem hemorragia vaginal inexplicável.
- se tem cancro dos ovários, do útero ou da mama.
- se tem uma afecção que impossibilitaria um embarazo normal, como menopausa precoce, malformações dos órgãos sexuais ou tumores benignos no útero.
Não utilize este medicamento se alguma das condições acima se aplica a si. Se não tiver certeza, consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar este medicamento.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Pergoveris.
Porfiria
Consulte o seu médico antes de iniciar o tratamento, se si ou algum membro da sua família padece porfiria (uma incapacidade para degradar as porfirinas que pode transmitir-se de pais a filhos).
Informar imediatamente ao seu médico se:
- a sua pele se torna frágil e lhe saem bolhas com facilidade, especialmente nas zonas expostas ao sol com frequência.
- tem dor de estômago, de braços ou pernas.
Nestes casos, o seu médico pode recomendar-lhe interromper o tratamento.
Síndrome de hiperestimulação ovárica (SHO)
Este medicamento estimula os seus ovários, o que aumenta o risco de experimentar síndrome de hiperestimulação ovárica (SHO). Isso ocorre quando os seus folículos se desenvolvem demasiado e se convertem em quistes de grande tamanho. Se tiver dor na região pélvica, aumenta de peso rapidamente, tem náuseas ou vómitos ou dificuldade para respirar, consulte imediatamente com o seu médico, que pode ordenar-lhe interromper o tratamento (ver na secção 4, em “Efeitos adversos mais graves”).
Em caso de que não ovule e se respeitam a dose e a posologia recomendadas, o SHO grave é menos provável que ocorra. O tratamento com Pergoveris raramente causa SHO grave. Isso é mais provável se for administrado o medicamento que se usa para a maturação folicular final (que contém gonadotropina coriónica humana, hCG) (ver detalhes na secção 3, em “Quantidade a usar”). Em caso de desenvolver SHO, o seu médico pode não lhe prescrever hCG neste ciclo de tratamento e aconselhá-lo a que se abstenha de realizar o coito ou que utilize um método anticonceptivo de barreira durante pelo menos 4 dias.
O seu médico assegurará um controlo cuidadoso da resposta ovárica, mediante ecografias e análises de sangue (determinações do estradiol), antes e durante o tratamento.
Embarazo múltiplo
Se usar Pergoveris, tem um risco mais alto de engravidar de mais de um bebé de cada vez (“embarazo múltiplo”, geralmente gêmeos) do que se engravidar por concepção natural. O embarazo múltiplo pode causar complicações médicas para si e os seus bebés. Pode reduzir o risco de embarazo múltiplo usando a dose correcta de Pergoveris às horas correctas.
Para minimizar o risco de embarazo múltiplo, recomenda-se realizar ecografias e análises de sangue.
Aborto
Se se submeter a estimulação dos seus ovários para produzir óvulos, a probabilidade de ter um aborto é maior do que na média das mulheres.
Embarazo ectópico
As mulheres que já sofreram alguma vez bloqueio ou danos das trompas de Falópio (enfermidade tubárica) apresentam risco de embarazo com implantação do embrião fora do útero (embarazo ectópico). Isso é assim tanto se o embarazo for por concepção espontânea como se for logrado mediante tratamentos de fertilidade.
Problemas de coagulação do sangue (episódios tromboembólicos)
Consulte o seu médico antes de começar a usar Pergoveris se si ou algum membro da sua família já sofreu alguma vez coágulos de sangue na perna ou no pulmão, infarto de miocárdio ou acidente vascular cerebral. Pode ter um risco mais alto de sofrer coágulos de sangue graves ou de agravar os coágulos existentes com o tratamento com Pergoveris.
Tumores dos órgãos sexuais
Foram comunicados tumores nos ovários e noutros órgãos sexuais, tanto benignos como malignos, em mulheres que foram submetidas a múltiplas pautas para o tratamento da infertilidade.
Reacções alérgicas
Foram comunicados casos isolados de reacções alérgicas não graves a Pergoveris. Se si já teve alguma vez este tipo de reacção com um medicamento semelhante, consulte o seu médico antes de começar a usar Pergoveris.
Crianças e adolescentes
Pergoveris não deve ser utilizado em crianças e adolescentes menores de 18 anos de idade.
Outros medicamentos e Pergoveris
Informar o seu médico ou farmacêutico se está a utilizar, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento.
Não use Pergoveris com outros medicamentos na mesma injeção, excepto com folitropina alfa, se lhe for prescrito pelo seu médico.
Embarazo e amamentação
Não use Pergoveris se estiver grávida ou em período de amamentação.
Condução e uso de máquinas
Não se espera que este medicamento afete a sua capacidade para conduzir ou utilizar máquinas.
Pergoveris contém sódio
Pergoveris contém menos de 1 mmol de sódio (23 mg) por dose; isto é, é essencialmente “isento de sódio”.
3. Como usar Pergoveris
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Uso deste medicamento
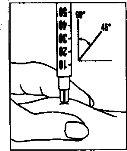
- Pergoveris está desenhado para ser injectado justo debaixo da pele (por via subcutânea). Para minimizar a irritação cutânea, seleccione um local de injeção diferente cada dia.
- Apresenta-se em forma de pó e líquido, que deve misturar e usar de forma imediata.
- O seu médico ou enfermeiro ensinar-lhe-á a preparar e injectar-se este medicamento. Eles supervisionarão a sua primeira injeção.
- Se estiverem de acordo com que possa administrar-se Pergoveris com segurança, em diante poderá preparar e injectar-se o medicamento si mesma em casa. Quando o fizer, leia e siga atentamente as instruções descritas a seguir na secção “Como preparar e usar o pó e diluente de Pergoveris”.
Quantidade a usar
A dose inicial habitual é de 1 frasco de Pergoveris cada dia.
- Em função da resposta, o seu médico pode decidir adicionar diariamente uma dose de um preparado de folitropina alfa autorizado à injeção de Pergoveris. Neste caso, normalmente a dose de folitropina alfa é incrementada cada 7 ou 14 dias em 37,5-75 UI.
- O tratamento continua até que se obtenha a resposta desejada. Isso acontece quando desenvolveu um folículo adequado, avaliado mediante ecografias e análises de sangue.
- Podem ser necessárias até cinco semanas.
Quando se obtém a resposta desejada, será administrada uma injeção única de gonadotropina coriónica humana (hCG) de 24 a 48 horas após a sua última injeção de Pergoveris. O melhor momento para manter relações sexuais é o mesmo dia da injeção de hCG e ao dia seguinte. Como alternativa, também pode ser realizada inseminação intrauterina ou outro procedimento de reprodução medicamente assistida, a critério do seu médico.
Se se obtém uma resposta excessiva, será interrompido o tratamento e não lhe será administrada hCG (ver na secção 2, em “Síndrome de hiperestimulação ovárica (SHO)”). Neste caso, o seu médico prescrever-lhe-á uma dose de folitropina alfa mais baixa no ciclo seguinte.
Como preparar e usar o pó e diluente de Pergoveris
Antes de começar a preparação, leia primeiro integralmente estas instruções:
Coloque a injeção à mesma hora cada dia.
- Lave as mãos e procure um local limpo
- É importante que as suas mãos e os materiais que utilize estejam o mais limpos possível
- Um local adequado é uma mesa limpa ou uma superfície da cozinha
- Reúna e disponha tudo o que vai precisar
- 1 frasco que contém o pó de Pergoveris
- 1 frasco que contém a água para preparações injectáveis (diluente)
Não se fornece no envase:
- 2 torundas embebidas em álcool
- 1 seringa vazia para injeção
- 1 agulha para a preparação
- 1 agulha fina para a injeção debaixo da pele
- um recipiente para objetos cortantes para deitar fora com precaução os frascos de vidro e as agulhas
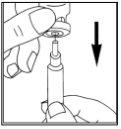
- Preparação da solução
|
|
|
|
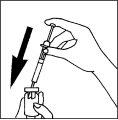
- Preparação da seringa para a injeção
|
|
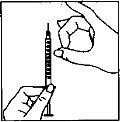
- Injeção da dose
|
|
- Depois da injeção
Deite fora todo o material: Uma vez finalizada a injeção, deite fora imediatamente todas as agulhas e frascos vazios no recipiente para objetos cortantes. Deve deitar fora qualquer porção da solução não utilizada.
Se usar mais Pergoveris do que deve
Desconhecem-se os efeitos de uma sobredose de Pergoveris; no entanto, pode esperar-se que se produza um SHO. Não obstante, isso só ocorrerá se for administrada hCG (ver na secção 2, em “Síndrome de hiperestimulação ovárica (SHO)”).
Se esquecer de usar Pergoveris
Não use uma dose dupla para compensar as doses esquecidas. Contacte o seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram.
Efeitos adversos mais graves
Consulte o seu médico imediatamente se detectar algum dos efeitos adversos que se listam a seguir. O médico poderá dizer-lhe que deixe de usar Pergoveris.
Reacções alérgicas
As reacções alérgicas, como erupção cutânea, rubor da pele, bolhas, inchaço do rosto com dificuldade para respirar, por vezes podem ser graves. Este efeito adverso é muito raro.
Síndrome de hiperestimulação ovárica (SHO)
- Dor pélvica, acompanhada de náuseas ou vómitos. Pode ser um sintoma do síndrome de hiperestimulação ovárica (SHO). É possível que os seus folículos tenham reagido de forma excessiva ao tratamento e se tenham desenvolvido quistes ováricos ou bolsas de líquido de grande tamanho (ver na secção 2 em “Síndrome de hiperestimulação ovárica (SHO)”). Este efeito adverso é frequente. Se lhe acontecer isto, o seu médico terá que examiná-la o mais breve possível.
- O SHO pode agravar-se com ovários claramente aumentados de tamanho, diminuição da produção de urina, aumento de peso, dificuldade para respirar e/ou possível acumulação de líquido no abdómen ou no peito. Este efeito adverso é pouco frequente (pode afectar até 1 de cada 100 pessoas).
- As complicações do SHO como torção ovárica ou coagulação do sangue ocorrem raramente (podem afectar até 1 de cada 1 000 pessoas).
- Os problemas de coagulação do sangue graves (episódios tromboembólicos), normalmente com SHO grave, ocorrem muito raramente. Isto poderia causar dor no peito, sensação de falta de ar, acidente vascular cerebral ou infarto de miocárdio. Em casos raros isto também poderia acontecer independentemente do SHO (ver na secção 2 em “Problemas de coagulação do sangue (episódios tromboembólicos)”).
Outros efeitos adversos
Muito frequentes (podem afectar mais de 1 de cada 10 pessoas)
- bolsas de líquido no interior dos ovários (quistes ováricos)
- dor de cabeça
- reacções locais no local de injeção como dor, picar, hematomas, inchaço ou irritação.
Frequentes (podem afectar até 1 de cada 10 pessoas)
- diarreia
- dor no peito
- náuseas ou vómitos
- dor abdominal ou pélvica
- cãibras ou distensão abdominal
Muito raros (podem afectar até 1 de cada 10 000 pessoas)
- o asma pode piorar.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Pergoveris
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece nos frascos e na caixa após CAD. A data de validade é o último dia do mês que se indica.
Não conserve a uma temperatura superior a 25 ºC. Conservar no embalagem original para protegê-lo da luz.
O medicamento deve ser administrado imediatamente após a reconstituição.
Não utilize Pergoveris se observar sinais visíveis de deterioração.
A solução reconstituída não deve ser administrada se contém partículas ou não é límpida.
Os medicamentos não devem ser jogados nos deságues nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Pergoveris
Os princípios ativos são folitropina alfa e lutropina alfa.
- Um frasco contém 150 UI de folitropina alfa (equivalente a 11 microgramas) e 75 UI de lutropina alfa (equivalente a 3 microgramas).
- Após a reconstituição, cada ml da solução contém 150 UI de folitropina alfa e 75 UI de lutropina alfa por mililitro.
Os outros componentes são:
- Sacarose, hidrogenofosfato de disódio dihidrato, dihidrogenofosfato de sódio monohidrato, metionina e polissorbato 20, assim como ácido fosfórico concentrado e hidróxido sódico para ajuste do pH.
Aspecto do produto e conteúdo do envase
- Pergoveris apresenta-se como um pó e solvente para solução injetável.
- O pó é uma pastilha liofilizada de cor branca a esbranquiçada em um frasco de vidro com tampa de borracha de bromobutilo que contém 150 UI (equivalente a 11 microgramas) de folitropina alfa e 75 UI (equivalente a 3 microgramas) de lutropina alfa.
- O solvente é um líquido claro e incolor em um frasco de vidro que contém 1 ml de água para preparações injetáveis.
- Pergoveris apresenta-se em envases que contêm 1, 3 e 10 frascos de pó junto com o mesmo número de frascos de solvente (1, 3 e 10 frascos). Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Titular da autorização de comercialização
Merck Europe B.V., Gustav Mahlerplein 102, 1082 MA Amsterdam, Países Baixos
Responsável pela fabricação
Merck Serono S.p.A, Via delle Magnolie 15 (Zona industrial), 70026 Modugno (Bari), Itália
Data da última revisão deste prospecto: {MM/AAAA}.
Outras fontes de informação
A informação detalhada deste medicamento está disponível na página web da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a PERGOVERIS 150 UI/75 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVELForma farmacêutica: INJETÁVEL, 300 UI/150 UISubstância ativa: combinationsFabricante: Merck Europe B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 450 UI/225 UISubstância ativa: combinationsFabricante: Merck Europe B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 900 UI/450 UISubstância ativa: combinationsFabricante: Merck Europe B.V.Requer receita médica
Alternativas a PERGOVERIS 150 UI/75 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a PERGOVERIS 150 UI/75 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Ukraine
Médicos online para PERGOVERIS 150 UI/75 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de PERGOVERIS 150 UI/75 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.