Como usar KESIMPTA 20 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDA
Introdução
Prospecto: informação para o paciente
Kesimpta 20 mg solução injetável em caneta precarregada
ofatumumab
Este medicamento está sujeito a acompanhamento adicional, o que agilizará a detecção de nova informação sobre a sua segurança. Pode contribuir comunicando os efeitos adversos que possa ter. A parte final da seção 4 inclui informação sobre como comunicar estes efeitos adversos.
Leia todo o prospecto detenidamente antes de começar a usar este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Kesimpta e para que é utilizado
- O que precisa saber antes de começar a usar Kesimpta
- Como usar Kesimpta
- Efeitos adversos possíveis
- Conservação de Kesimpta
- Conteúdo do envase e informação adicional
1. O que é Kesimpta e para que é utilizado
O que é Kesimpta
Kesimpta contém o princípio ativo ofatumumab. Ofatumumab pertence a um grupo de medicamentos conhecidos como anticorpos monoclonais.
Para que é utilizado Kesimpta
Kesimpta é utilizado para tratar adultos com formas recorrentes de esclerose múltipla (EMR).
Como funciona Kesimpta
Kesimpta funciona mediante a união a uma diana conhecida como CD20 que está na superfície dos linfócitos B. Os linfócitos B são um tipo de glóbulos brancos que fazem parte do sistema imunológico (as defesas do organismo). Na esclerose múltipla, o sistema imunológico ataca a camada protectora em torno das células nervosas. Os linfócitos B estão implicados neste processo. Kesimpta dirige-se aos linfócitos B e os elimina. Desta forma reduz a possibilidade de um surto, alivia os sintomas e reduz a velocidade de progressão da doença.
2. O que precisa saber antes de começar a usar Kesimpta
Não use Kesimpta
- se é alérgico ao ofatumumab ou a algum dos outros componentes deste medicamento (incluídos na seção 6).
- se lhe foram diagnosticados problemas graves no seu sistema imunológico.
- se tem uma infecção grave.
- se tem cancro.
Advertências e precauções
Consulte o seu médico antes de começar a usar Kesimpta
- Kesimpta pode provocar que o vírus da hepatite B volte a ativar-se. O seu médico realizará uma análise de sangue para comprovar se tem risco de infecção por hepatite B. Se o resultado mostrar que teve hepatite B ou que é portador do vírus da hepatite B, o seu médico pedirá que visite um especialista.
- Antes de iniciar o tratamento com Kesimpta, o seu médico pode comprovar o seu sistema imunológico.
- Se tem uma infecção, o seu médico pode decidir que não pode usar Kesimpta ou que deve adiar o tratamento com Kesimpta até que a infecção se tenha resolvido.
- O seu médico comprovará se precisa de alguma vacina antes de iniciar o tratamento com Kesimpta. Se necessitar de um tipo de vacina conhecido como vacina viva ou viva atenuada, esta deve ser administrada pelo menos 4 semanas antes de começar o tratamento com Kesimpta. Outros tipos de vacinas devem ser administrados pelo menos 2 semanas antes de começar o tratamento com Kesimpta.
Enquanto usa Kesimpta
Informa o seu médico:
- se apresenta uma reação geral relacionada com a injeção ou uma reação local no local da injeção. Estes são os efeitos adversos mais comuns do tratamento com Kesimpta e são descritos na seção 4. Normalmente ocorrem no prazo de 24 horas após ter injetado Kesimpta, especialmente após a primeira injeção. A primeira injeção deve ser realizada sob a orientação de um profissional de saúde.
- se tem uma infecção. Pode contrair infecções com mais facilidade ou se já apresenta uma infecção, esta poderia piorar. Isto deve-se a que as células imunológicas sobre as quais actua Kesimpta também ajudam a combater infecções. As infecções poderiam ser graves e, por vezes, até de ameaça para a vida.
- se tem previsto vacinar-se. O seu médico indicará se a vacina que necessita é uma vacina viva, uma vacina viva atenuada ou outro tipo de vacina. Durante o tratamento com Kesimpta não lhe devem ser administradas vacinas vivas ou vivas atenuadas, porque pode provocar-lhe uma infecção. Outros tipos de vacinas podem não funcionar tão bem se forem recebidos durante o tratamento com Kesimpta.
Informa o seu médico imediatamente se apresenta algum dos seguintes sintomas durante o tratamento com Kesimpta, porque poderiam ser sinais de uma doença grave:
- se apresenta erupção cutânea, urticária, dificuldade em respirar, inchaço do rosto, pálpebras, lábios, boca, língua ou garganta, opressão no peito, ou se sente débil. Estes podem ser sinais ou sintomas de uma reação alérgica.
- se pensa que a sua esclerose múltipla está a piorar (p. ex. apresenta fraqueza ou alterações na visão) ou se nota algum sintoma novo ou incomum. Estes efeitos podem ser indicativos de um distúrbio raro do cérebro conhecido como leucoencefalopatia multifocal progressiva (LMP), que é provocado pela infecção de um vírus.
Crianças e adolescentes
Não administre este medicamento a crianças e adolescentes menores de 18 anos, porque Kesimpta ainda não foi estudado neste grupo etário.
Outros medicamentos e Kesimpta
Informa o seu médico ou farmacêutico se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Em especial, informa o seu médico ou farmacêutico:
- se está a tomar, tomou recentemente ou pode ter que tomar medicamentos que afetam o sistema imunológico. Isto deve-se a que se poderiam somar os efeitos sobre o sistema imunológico.
- se tem previsto vacinar-se (ver anteriormente “Advertências e Precauções”).
Gravidez e amamentação
Se está grávida ou em período de amamentação, acha que pode estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento.
Gravidez
Deve evitar engravidar enquanto usa Kesimpta e durante os 6 meses posteriores à data em que deixar de usá-lo.
Se é uma mulher em idade fértil deve utilizar um método anticonceptivo eficaz durante o tratamento e durante 6 meses após interromper o tratamento com Kesimpta. Consulte com o seu médico as opções disponíveis.
Se engravidar ou acha que pode estar grávida durante o tratamento ou durante os 6 meses posteriores à última dose, informa o seu médico imediatamente. O seu médico informá-lo-á dos riscos potenciais de Kesimpta na gravidez. Isto deve-se a que Kesimpta pode reduzir o número de células do sistema imunológico (linfócitos B) tanto na mãe como no feto. O seu médico deve comunicar a sua gravidez à Novartis. Você também pode comunicar a sua gravidez contactando o representante local da Novartis (ver seção 6), além de contactar o seu médico.
Amamentação
Kesimpta pode passar para o leite materno. Consulte com o seu médico os benefícios e riscos antes de dar o peito enquanto usa Kesimpta.
Vacinação dos bebés recém-nascidos
Consulte o seu médico ou farmacêutico antes de vacinar o seu bebé recém-nascido se você usou Kesimpta durante a gravidez (ver anteriormente “Advertências e precauções”).
Condução e uso de máquinas
Não é provável que Kesimpta afete a sua capacidade para conduzir e utilizar máquinas.
Kesimpta contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por dose; isto é, essencialmente “isento de sódio”.
Kesimpta contém polissorbato 80
Este medicamento contém 0,08 mg de polissorbato 80 por dose. Os polissorbatos podem causar reações alérgicas. Informa o seu médico se tem alguma alergia conhecida.
3. Como usar Kesimpta
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Kesimpta é administrado mediante injeção subcutânea (injeção debaixo da pele).
A primeira injeção deve ser realizada sob a orientação de um profissional de saúde.
As canetas precarregadas de Kesimpta são para um só uso.
Pode consultar as instruções detalhadas sobre como injetar Kesimpta no apartado “Instruções de uso de Kesimpta em Caneta Sensoready” no final deste prospecto.
‘Código QR a incluir’ + www.kesimpta.eu
Pode usar Kesimpta em qualquer momento do dia (manhã, tarde ou noite).
Quanto Kesimpta e com que frequência é administrado
Não exceda a dose que o seu médico lhe prescreveu.
- A dosagem inicial é de 20 mg de Kesimpta que é administrado no primeiro dia de tratamento (Semana 0) e após 1 semana e 2 semanas (Semana 1 e Semana 2). Após estas primeiras 3 injeções, na semana seguinte não se deve administrar nenhuma injeção (Semana 3).
- A dose recomendada é de 20 mg de Kesimpta uma vez por mês, começando na Semana 4.
Tempo | Dose |
Semana 0 (primeiro dia do tratamento) | 20 mg |
Semana 1 | 20 mg |
Semana 2 | 20 mg |
Semana 3 | Nenhuma injeção |
Semana 4 | 20 mg |
Depois, cada mês | 20 mg |
Durante quanto tempo usar Kesimpta
Continue a usar Kesimpta cada mês durante o tempo que o seu médico indicar.
O seu médico controlará periodicamente o estado da sua doença para comprovar se o tratamento tem o efeito desejado.
Se tiver alguma dúvida sobre quanto tempo deve usar Kesimpta, pergunte ao seu médico, farmacêutico ou enfermeiro.
Se usar mais Kesimpta do que deve
Se se administrar demasiado Kesimpta, informa o seu médico imediatamente.
Se esquecer de usar Kesimpta
Para obter o benefício completo de Kesimpta, é importante que se ponha cada injeção quando lhe corresponda.
Se esquecer de se pôr uma injeção de Kesimpta, deve administrá-la o mais breve possível. Não espere até a próxima dose prevista. Os tempos de administração das próximas injeções devem ser então calculados desde o dia em que se injetou esta dose e não com base no calendário original (ver também o apartado anterior “Quanto Kesimpta e com que frequência é administrado”).
Se interromper o tratamento com Kesimpta
Não interrompa o tratamento com Kesimpta nem altere a sua dose sem comentar antes com o seu médico.
Alguns efeitos adversos podem estar relacionados com níveis baixos de linfócitos B no sangue. Após interromper o tratamento com Kesimpta, os níveis de linfócitos B no sangue irão aumentando gradualmente até atingir níveis normais. Isto pode durar vários meses, durante os quais pode experimentar ainda alguns dos efeitos adversos descritos neste prospecto.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
A seguir são mencionados os efeitos adversos de Kesimpta. Se algum destes efeitos adversos o afetar de forma grave, informa o seu médico, farmacêutico ou enfermeiro.
Muito frequentes(podem afetar mais de 1 de cada 10 pacientes)
- infecções das vias respiratórias altas, com sintomas como irritação da garganta e gotejamento nasal
- reações relacionadas com a injeção, tais como febre, dor de cabeça, dor muscular, arrepios e cansaço - estes normalmente aparecem nas 24 horas seguintes a uma injeção de Kesimpta e principalmente após a primeira injeção
- infecções das vias urinárias
- reações no local da injeção, tais como vermelhidão, dor, picazão e inchaço no local da injeção
Frequentes(podem afetar até 1 de cada 10 pacientes)
- descida no nível sanguíneo de uma proteína conhecida como imunoglobulina M, que ajuda a proteger contra infecções
- herpes oral
- náuseas, vómitos (foram comunicados associados a reações relacionadas com a injeção)
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis)
- reações alérgicas, com sintomas como erupção cutânea, urticária, dificuldade em respirar, inchaço do rosto, pálpebras, lábios, boca, língua ou garganta, opressão no peito, ou sensação de fraqueza
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informação sobre a segurança deste medicamento.
5. Conservação de Kesimpta
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa após CAD e na etiqueta após EXP. A data de validade é o último dia do mês que se indica.
Conserva a(s) caneta(s) precarregada(s) no embalagem exterior para protegê-la(s) da luz. Conserva em frigorífico (entre 2 °C e 8 °C). Não congele.
Em caso necessário, Kesimpta pode ser deixado fora do frigorífico durante um período único de até 7 dias a temperatura ambiente (não superior a 30 °C). Se não for utilizado durante este período, Kesimpta pode voltar a ser colocado no frigorífico durante um máximo de 7 dias.
Não utilize este medicamento se observar que a solução contém partículas visíveis ou está turva.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Kesimpta
- O princípio ativo é o ofatumumab. Cada caneta precarregada contém 20 mg de ofatumumab.
- Os outros componentes são L-arginina, acetato de sódio trihidrato, cloreto de sódio, polissorbato 80 (E 433), edetato dissódico dihidrato, ácido clorídrico (para ajuste do pH) e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Kesimpta solução injetável é uma solução entre transparente e ligeiramente opalescente, e entre incolor e amarela-ligeiramente amarronada.
Kesimpta está disponível em envases unitários que contêm 1 Caneta Sensoready precarregada e em envases múltiplos que se compõem de 3 caixas, com 1 Caneta Sensoready precarregada em cada uma.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título de autorização de comercialização
Novartis Europharm Limited
Edifício Vista
Elm Park, Merrion Road
Dublin 4
Irlanda
Responsável pela fabricação
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Alemanha
Novartis Farmacêutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Espanha
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemanha
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lituânia SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxemburgo Novartis Pharma N.V. Tel: +32 2 246 16 11 |
República Tcheca Novartis s.r.o. Tel: +420 225 775 111 | Hungria Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Dinamarca Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Alemanha Novartis Pharma GmbH Tel: +49 911 273 0 | Países Baixos Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estônia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Noruega Novartis Norge AS Tlf: +47 23 05 20 00 |
Grécia Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Áustria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Espanha Novartis Farmacêutica, S.A. Tel: +34 93 306 42 00 | Polônia Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
França Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croácia Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | Romênia Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Irlanda Novartis Ireland Limited Tel: +353 1 260 12 55 | Eslovênia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Islândia Vistor hf. Sími: +354 535 7000 | República Eslovaca Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Itália Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finlândia Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Chipre Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Suécia Novartis Sverige AB Tel: +46 8 732 32 00 |
Letônia SIA Novartis Baltics Tel: +371 67 887 070 |
Data da última revisão deste prospecto:
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: https://www.ema.europa.eu.




Instruções de uso de Kesimpta em caneta Sensoready
É importante que entenda e siga estas instruções de uso antes de se injetar Kesimpta. Se tiver alguma dúvida, consulte seu médico, farmacêutico ou enfermeiro antes de usar Kesimpta pela primeira vez.
Lembre-se:
- Não usea caneta se o lacre da caixa ou o lacre da caneta estiverem quebrados. Mantenha a caneta dentro da caixa lacrada até que esteja pronto para usá-la.
- Não agitea caneta.
- Se a caneta cair, não a usese parecer que está danificada, ou se a caneta caiu sem o bico colocado.
- Elimine a caneta usada imediatamente após usá-la. Não reutilize uma caneta. Veja o item “Como devo eliminar a caneta Sensoready de Kesimpta usada?” no final destas Instruções de Uso.
Como devo armazenar Kesimpta?
- Mantenha a caixa da caneta na geladeira entre 2 °C e 8 °C.
- Mantenha a caneta no embalagem original para protegê-la da luz, até que esteja pronto para usá-la.
- Não congelea caneta.
Mantenha Kesimpta fora do alcance e da vista das crianças.
Partes da caneta Sensoready de Kesimpta (ver Imagem A):
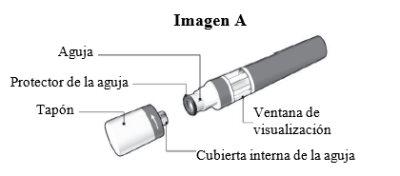
A caneta Sensoready de Kesimpta é mostrada com o bico removido. Não remova o bico até que esteja pronto para a injeção.
O que você precisa para a injeção:
Incluído na caixa:
|
|
Não incluído na caixa (ver Imagem C):
|
|
Veja o item “Como devo eliminar a caneta Sensoready de Kesimpta usada?” no final destas Instruções de Uso.
Antes da injeção:
Retire a caneta da geladeira de 15 a 30 minutos antes da injeçãopara que atinja a temperatura ambiente.
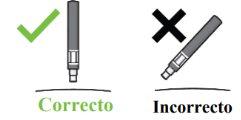
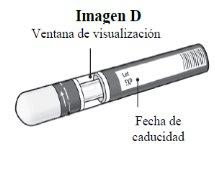
Passo 1. Verificações de segurança importantes antes de realizar a injeção (ver Imagem D):
Não usea caneta se o líquido contiver partículas visíveis ou estiver turvo. Pode ser que veja uma pequena bolha de ar, o que é normal.
Entre em contato com o farmacêutico ou profissional de saúde se a caneta não atender a alguma dessas verificações. |
|
|
|
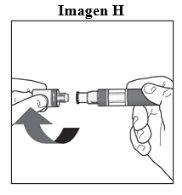
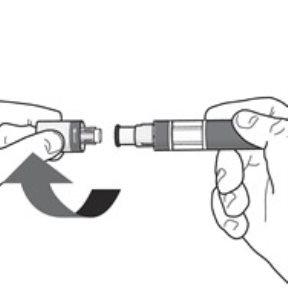
Sua injeção Passo 4. Remova o bico:
Pode ser que observe algumas gotas de medicamento saindo da agulha. Isso é normal. |
|
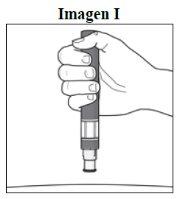
Passo 5. Segure a caneta:
|
|
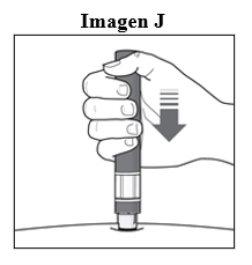
Passo 6. Inicie a injeção:
|
|
Passo 7. Finalize a injeção:
|
|
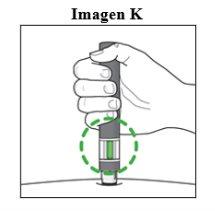
Importante: Durante a injeçãoouvirá 2 cliques intensos:
Deve segurar a caneta firmemente pressionada contra a pele até que o indicador verdepreencha a janela e pare de se mover. |
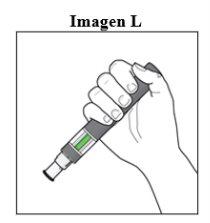
Depois da injeção:
|
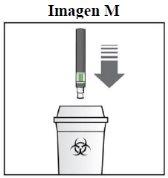
Como devo eliminar a caneta Sensoready de Kesimpta usada?
Passo 8. Elimine a caneta Sensoread de Kesimpta:
Mantenha o contenedor de eliminação de objetos pontiagudos fora do alcance das crianças. |
|
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a KESIMPTA 20 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDAForma farmacêutica: PERFURAÇÃO INJETÁVEL, 120 mg (80 mg/kg) belimumabeSubstância ativa: belimumabFabricante: Glaxosmithkline (Ireland) LimitedRequer receita médicaForma farmacêutica: INJETÁVEL, 200 mgSubstância ativa: belimumabFabricante: Glaxosmithkline (Ireland) LimitedRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 400 mg (80 mg/kg) belimumabeSubstância ativa: belimumabFabricante: Glaxosmithkline (Ireland) LimitedRequer receita médica
Médicos online para KESIMPTA 20 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de KESIMPTA 20 mg SOLUÇÃO INJETÁVEL EM CANETA PREENCHIDA – sujeita a avaliação médica e regras locais.





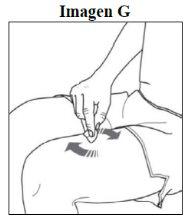
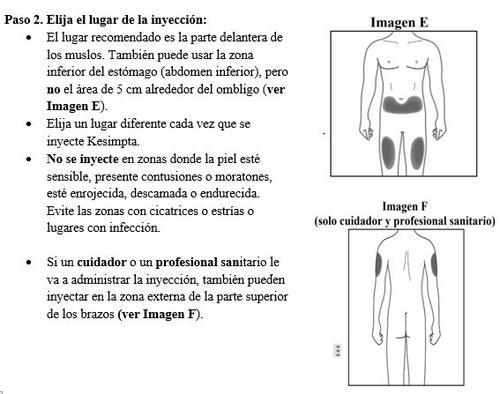
 Passo 3. Limpe o local da injeção:
Passo 3. Limpe o local da injeção: