INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA

Pergunte a um médico sobre a prescrição de INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA

Como usar INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA
Introdução
Prospecto: informação para o utilizador
Infanrix-IPV, suspensão injetável em seringa pré-carregada
Vacina antidiftérica, antitetânica, antipertussis (componente acelular) e antipoliomielítica (inativada) (adsorvida)
Leia todo o prospecto detenidamente antes de o seu filho começar a receber esta vacina, porque contém informações importantes para si.
|
Conteúdo do prospecto
- O que é Infanrix-IPV e para que é utilizado
- O que precisa saber antes de o seu filho receber Infanrix-IPV
- Como é administrado Infanrix-IPV
- Efeitos adversos possíveis
- Conservação de Infanrix-IPV
- Conteúdo do envase e informação adicional
1. O que é Infanrix-IPV e para que é utilizado
Infanrix-IPV é uma vacina que é utilizada como dose de reforço para proteger o seu filho contra 4 doenças:
- Difteria- uma infecção bacteriana grave que afeta principalmente as vias respiratórias e, algumas vezes, a pele. As vias respiratórias incham-se, causando problemas respiratórios graves e, algumas vezes, asfixia. A bactéria também libera uma toxina que pode causar danos nos nervos, problemas cardíacos e até a morte.
- Tétano- a bactéria do tétano penetra no organismo através de cortes, arranhões ou feridas na pele. As feridas com maior probabilidade de infecção com tétano são as queimaduras, fraturas, feridas profundas ou as feridas que contêm sujeira, pó, excrementos de cavalo ou estilhaços de madeira. A bactéria libera uma toxina que pode causar rigidez muscular, espasmos musculares dolorosos, convulsões e até a morte. Os espasmos musculares podem ser suficientemente fortes para causar fraturas ósseas da coluna vertebral.
- Tosse ferina (pertussis)- uma doença altamente contagiosa que afeta as vias respiratórias. Causa tosse grave que pode produzir problemas na respiração. Muitas vezes a tosse tem um som semelhante a um “uivo”. A tosse pode durar de um a dois meses ou mais. A tosse ferina também pode causar infecções de ouvido, infecções de peito (bronquite) que podem prolongar-se no tempo, infecções pulmonares (pneumonia), convulsões, lesão cerebral e até a morte.
- Polio- uma infecção produzida por um vírus. Muitas vezes a polio é apenas uma doença leve, embora algumas vezes possa ser muito grave e causar danos permanentes ou até a morte. A polio pode fazer com que os músculos sejam incapazes de se mover (paralisia). Isso inclui os músculos necessários para respirar e caminhar. Os braços ou as pernas afetados pela doença podem torcer-se (deformar-se) de forma dolorosa.
Infanrix-IPV é para crianças de 16 meses a 13 anos (ambos incluídos). Não está indicada para pessoas maiores de 14 anos.
Como funciona Infanrix-IPV
- Infanrix-IPV ajuda o organismo a desenvolver sua própria proteção (anticorpos). Isso protegerá o seu filho contra essas doenças.
- A vacina não pode produzir as doenças das quais protege o seu filho.
2. O que precisa saber antes de o seu filho receber Infanrix-IPV
Não se deve administrar Infanrix-IPV
- Se o seu filho é alérgico aos princípios ativos ou a algum dos outros componentes da vacina (incluídos na seção 6) ou à neomicina ou à polimixina (tipos de antibióticos) ou ao formaldeído. Os sinais de uma reação alérgica podem incluir erupção na pele com coceira, dificuldade para respirar e inchaço do rosto ou língua.
- Se o seu filho teve qualquer reação alérgica a vacinas contra a difteria, tétano, tosse ferina ou polio.
- Se o seu filho teve problemas do sistema nervoso (encefalopatia) nos 7 dias seguintes à administração de uma vacina contra a tosse ferina.
- Se o seu filho tem uma infecção grave com febre (superior a 38°C). Uma infecção leve, como um resfriado, não deve ser um problema, mas consulte primeiro o seu médico.
Não se deve administrar Infanrix-IPV se alguma das situações acima afetar o seu filho. Se não tiver certeza, fale com o seu médico ou farmacêutico antes de o seu filho receber Infanrix-IPV.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de o seu filho receber Infanrix-IPV se:
- após uma administração anterior de Infanrix-IPV ou de outra vacina contra a tosse ferina, o seu filho teve qualquer problema, especialmente:
- febre (superior a 40°C) nas 48 horas posteriores à vacinação
- colapso ou estado semelhante ao “choque” nas 48 horas seguintes à vacinação
- choro inconsolável, persistente por 3 horas ou mais de duração, produzido nas 48 horas seguintes à vacinação
- convulsões, com ou sem febre, nos 3 dias seguintes à vacinação
- o seu filho sofre de uma doença cerebral não diagnosticada ou progressiva ou epilepsia não controlada. A vacina deve ser administrada uma vez controlada a doença
- o seu filho tem algum problema hemorrágico ou aparecem cardenales com facilidade
- o seu filho tem tendência a sofrer convulsões causadas por febre ou se tem antecedentes familiares de convulsões por febre
- o seu filho tem problemas com o sistema imunológico (incluindo infecção por VIH). Neste caso, o seu filho pode ser vacinado com Infanrix-IPV. No entanto, a proteção obtida contra as infecções pode não ser tão elevada.
Antes ou depois de qualquer injeção, pode ocorrer um desmaio (especialmente em adolescentes), por isso deve informar o seu médico ou enfermeira se o seu filho desmaiou em ocasiões anteriores após a administração de uma injeção.
Se alguma das situações acima afetar o seu filho (ou não tiver certeza), fale com o seu médico ou farmacêutico antes de o seu filho receber Infanrix-IPV.
Outros medicamentos e Infanrix-IPV
Informar o seu médico ou farmacêutico se o seu filho está utilizando, utilizou recentemente ou possa ter que utilizar qualquer outro medicamento.
Em particular, informe o seu médico ou farmacêutico se o seu filho está utilizando algum dos seguintes:
- medicamentos ou outros tratamentos (como radioterapia) que afetem o sistema imunológico. O seu filho pode ser vacinado com Infanrix-IPV. No entanto, pode ser que Infanrix-IPV não funcione igual de bem. Se for possível, a vacina deve ser administrada uma vez que tenha finalizado este tratamento.
- Infanrix-IPV pode ser administrado ao mesmo tempo que outras vacinas. Serão utilizados locais de injeção distintos para cada vacina.
Gravidez e lactação
É pouco provável que Infanrix-IPV seja administrado a mulheres grávidas ou que estejam amamentando seus filhos. Isso ocorre porque a vacina está indicada apenas em crianças de 16 meses a 13 anos (ambos incluídos).
Não se recomenda a administração desta vacina durante a gravidez ou durante a amamentação.
Consulte o seu médico ou farmacêutico antes de utilizar qualquer medicamento.
Condução e uso de máquinas
É pouco provável que Infanrix-IPV seja administrado a pessoas que conduzam ou utilizem ferramentas ou máquinas. Isso ocorre porque a vacina está indicada apenas em crianças de 16 meses a 13 anos (ambos incluídos).
O seu filho pode sentir-se sonolento após a vacinação. Se isso ocorrer, o seu filho não deve conduzir, andar de bicicleta ou utilizar ferramentas ou máquinas.
Infanrix-IPV contém neomicina, polimixina (antibióticos), ácido para-aminobenzoico, fenilalanina, sódio, potássio e formaldeído.Não se deve administrar Infanrix-IPV ao seu filho se for alérgico a algum desses componentes. Por favor, consulte o seu médico se o seu filho teve uma reação alérgica a esses componentes.
Infanrix-IPV contém ácido para-aminobenzoico. Pode provocar reações alérgicas (possivelmente retardadas) e, excepcionalmente, broncoespasmo.
Esta vacina contém 0,036 microgramas de fenilalanina em cada unidade de dose. A fenilalanina pode ser prejudicial em caso de padecer fenilcetonúria (FCN), uma doença genética rara na qual a fenilalanina se acumula devido ao fato de o organismo não ser capaz de eliminá-la corretamente.
Esta vacina contém menos de 1 mmol de sódio (23 mg) por unidade de dose; isto é, essencialmente “isento de sódio”.
Esta vacina contém potássio, menos de 1 mmol (39 mg) por dose; isto é, essencialmente “isento de potássio”.
3. Como é administrado Infanrix-IPV
Quando é administrada a vacina
- O seu médico ou enfermeira o informará quando o seu filho deve receber esta vacina. Isso depende das recomendações oficiais.
Como é administrado Infanrix-IPV
- O seu filho receberá uma única injeção de Infanrix-IPV.
- Infanrix-IPV é injetado sempre em um músculo.
- A injeção será normalmente no músculo do ombro. No entanto, em crianças pequenas, pode ser administrada no músculo da coxa.
- A vacina nunca deve ser administrada em uma veia.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Efeitos adversos possíveis
Assim como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram. Com esta vacina podem ocorrer os seguintes efeitos adversos:
Reações alérgicas
Se o seu filho tiver uma reação alérgica, acuda a um médico imediatamente. Os sinais podem incluir:
- erupções cutâneas com coceira ou bolhas
- inchaço dos olhos e rosto
- dificuldade para respirar ou engolir
- diminuição repentina da tensão arterial
- perda de consciência.
Estes sinais geralmente começam pouco após receber a injeção. Leve o seu filho imediatamente ao médico se aparecerem após deixar a clínica. As reações alérgicas são muito raras (menos de 1 de cada 10.000 doses de vacina).
Consulte imediatamente o seu médico se o seu filho tiver algum dos seguintes efeitos adversos graves:
- colapso
- perda de consciência
- convulsões
Consulte imediatamente com o seu médico se observar alguma das situações descritas anteriormente. Estes efeitos adversos ocorreram com outras vacinas contra a tosse ferina. Normalmente aparecem nos 2 ou 3 dias posteriores à vacinação.
Outros efeitos adversos incluem:
Muito frequentes(podem ocorrer em mais de 1 de cada 10 doses da vacina): sensação de sonolência, dor de cabeça, perda de apetite, temperatura elevada de 38°C ou mais, dor, vermelhidão e inchaço no local da injeção, choro anormal, sensação de irritabilidade ou inquietude.
Frequentes(podem ocorrer até com 1 de cada 10 doses da vacina): diarreia, náuseas, vômitos, temperatura elevada de 39,5°C ou mais, sensação de mal-estar geral, erupção nodular no local da injeção, sensação de fraqueza.
Pouco frequentes(podem ocorrer até com 1 de cada 100 doses da vacina): alergias cutâneas ou erupções.
Raros(podem ocorrer até com 1 de cada 1.000 doses da vacina): inchaço dos gânglios do pescoço, axilas e virilha (linfadenopatia), tosse ou infecção de peito (bronquite), coceira, erupção nodular (urticária).
Muito raros(podem ocorrer até com 1 de cada 10.000 doses da vacina): sangramento ou aparecimento de cardenales mais facilmente do que o normal (trombocitopenia), parada passageira da respiração (apneia), inflamação do rosto, lábios, boca, língua ou garganta, podendo causar dificuldades para engolir ou respirar (edema angioneurótico), bolhas no local da injeção.
As doses de reforço de Infanrix-IPV podem aumentar o risco de reações no local da injeção. Algumas delas afetam todo o braço ou a perna na qual a injeção foi administrada. Estas reações normalmente começam a las 48 horas da injeção e desaparecem aos 4 dias.
Comunicação de efeitos adversos
Se o seu filho experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Infanrix-IPV
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Conservar em refrigerador (entre 2°C e 8°C).
- Conservar no embalagem original para protegê-lo da luz.
- Não congelar. A congelamento destrói a vacina.
- Não utilize este medicamento após a data de validade que aparece no embalagem. A data de validade é o último dia do mês que se indica.
- Os medicamentos não devem ser jogados fora pelos esgotos nem pela lixeira. Deposite os embalagens e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos embalagens e dos medicamentos que não precisa. Dessa forma, você ajudará a proteger o meio ambiente.
6. Conteúdo do embalagem e informação adicional
Composição de Infanrix-IPV
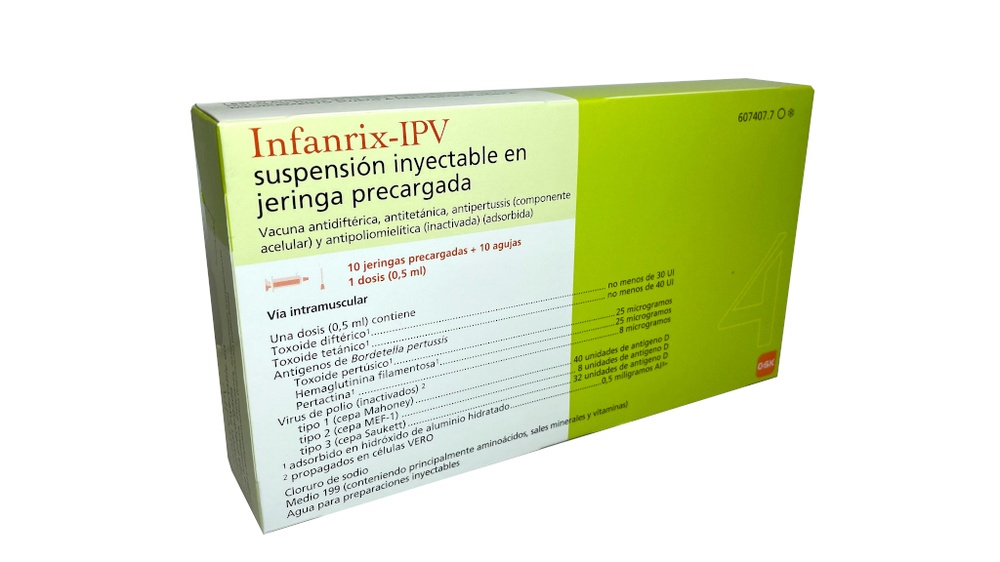
- Os princípios ativos são:
Toxoide diftérico1 não menos de 30 UI
Toxoide tetânico1 não menos de 40 UI
Antígenos de Bordetella pertussis
Toxoide pertussis1 25 microgramas
Hemaglutinina filamentosa1 25 microgramas
Pertactina1 8 microgramas
Vírus de polio (inativados)2
tipo 1 (cepa Mahoney) 40 unidades de antígeno D
tipo 2 (cepa MEF-1) 8 unidades de antígeno D
tipo 3 (cepa Saukett) 32 unidades de antígeno D
1 adsorvidos em hidróxido de alumínio hidratado 0,5 miligramas Al3+
2 propagados em células VERO
O hidróxido de alumínio é incluído nesta vacina como um adjuvante. Os adjuvantes são substâncias presentes em algumas vacinas para acelerar, melhorar e/ou prolongar os efeitos protectores da vacina.
- Os demais componentes são: cloreto de sódio, Meio 199 (contendo aminoácidos (incluindo fenilalanina), sais minerais (incluindo sódio e potássio) e vitaminas (incluindo ácido para-aminobenzoico) e outras substâncias), água para preparações injetáveis.
Aspecto do produto e conteúdo do embalagem
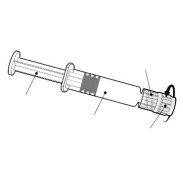
- Infanrix-IPV é uma suspensão injetável em seringa pré-carregada (0,5 ml).
- A suspensão é branca e ligeiramente leitosa.
- Infanrix-IPV está disponível em seringa pré-carregada de 1 dose com ou sem agulhas separadas; tamanhos de embalagem de 1 e 10.
- Pode ser que apenas alguns tamanhos de embalagens estejam comercializados.
Titular da autorização de comercialização e responsável pela fabricação
Titular da autorização de comercialização
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Espanha
Tel: +34 900 202 700
Responsável pela fabricação
GlaxoSmithKline Biologicals S.A.,
Rue de l' Institut 89
1330 Rixensart,Bélgica
Este medicamento está autorizado nos Estados membros do Espaço Econômico Europeu com os seguintes nomes:
Grécia, França, Portugal, Chipre: InfanrixTetra
República Checa, Estônia, Letônia, Lituânia, Noruega, República Eslovaca, Suécia: Infanrix Polio
Finlândia: Infanrix-Polio
Polônia, Espanha: Infanrix-IPV
Hungria: Infanrix IPV
Irlanda: IPV Infanrix
Itália: PolioInfanrix
Data da última revisão deste prospecto:abril 2023
Outras fontes de informação
A informação detalhada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais do setor sanitário:
Uma vez armazenada, pode ser observado um depósito branco e um sobrenadante claro. Isso não constitui um sinal de deterioração.
A seringa deve ser agitada bem para obter uma suspensão branca, turva e homogênea.
A suspensão deve ser examinada visualmente para observar se existe alguma partícula estranha e/ou variação do aspecto físico. Em caso de apreciar alguma dessas circunstâncias, descartar a vacina.
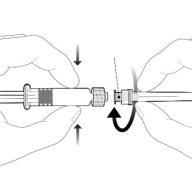
Instruções para a seringa pré-carregada
| Segure a seringa pelo corpo, não pelo émbolo. Desrosqueie o tampão da seringa girando-a em sentido contrário ao das agulhas do relógio. |
| Para inserir a agulha, conecte a base ao adaptador luer-locke gire-o um quarto de volta no sentido das agulhas do relógio até que sinta que se bloqueia. Não retire o émbolo da seringa do corpo. Se isso ocorrer, não administre a vacina. |
Eliminação de resíduos
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a normativa local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDAForma farmacêutica: INJETÁVEL, 0.5 mlSubstância ativa: diphtheria-pertussis-poliomyelitis-tetanusFabricante: Glaxosmithkline S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 0,5 mlSubstância ativa: diphtheria-pertussis-poliomyelitis-tetanusFabricante: Sanofi Winthrop IndustrieRequer receita médicaForma farmacêutica: INJETÁVEL, 1 doseSubstância ativa: diphtheria-pertussis-poliomyelitis-tetanusFabricante: Sanofi Winthrop IndustrieRequer receita médica
Alternativas a INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA em Polónia
Alternativa a INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA em Ukraine
Médicos online para INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de INFANRIX-IPV SUSPENSÃO PARA INJEÇÃO EM SERINGA PREENCHIDA – sujeita a avaliação médica e regras locais.