HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVEL

Pergunte a um médico sobre a prescrição de HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVEL

Como usar HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVEL
Introdução
Prospecto: Informação para o paciente
Hepcludex 2 mg pó para solução injetável
bulevirtida
Este medicamento está sujeito a acompanhamento adicional, o que agilizará a detecção de nova informação sobre sua segurança. Pode contribuir comunicando os efeitos adversos que possa ter. A parte final da seção 4 inclui informação sobre como comunicar esses efeitos adversos.
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Hepcludex e para que é utilizado
- O que precisa saber antes de começar a usar Hepcludex
- Como usar Hepcludex
- Possíveis efeitos adversos
- Conservação de Hepcludex
- Conteúdo do envase e informação adicional
- Guia para a injeção passo a passo
1. O que é Hepcludex e para que é utilizado
O que é Hepcludex
Hepcludex contém o princípio ativo bulevirtida, que é um medicamento antiviral.
Para que é utilizado Hepcludex
Hepcludex é utilizado para tratar a infecção a longo prazo (crônica) pelo vírus da hepatite delta (VHD) em adultos com doença hepática compensada (quando o fígado ainda funciona adequadamente). A infecção pelo vírus da hepatite delta causa inflamação do fígado.
Como actua Hepcludex
O VHD utiliza uma proteína concreta das células hepáticas para entrar nessas células. Bulevirtida, o princípio ativo deste medicamento, bloqueia a proteína e, assim, impede a entrada do VHD nas células hepáticas. Isso reduz a propagação do VHD no fígado e reduz a inflamação.
2. O que precisa saber antes de começar a usar Hepcludex
Não tome Hepcludex:
- se é alérgico a bulevirtida ou a algum dos outros componentes deste medicamento (incluídos na seção 6).
Se tiver dúvidas, consulte o seu médico antes de tomar este medicamento.
Advertências e precauções
Não interrompa o tratamento com Hepcludex a menos que o seu médico o aconselhe a fazê-lo. Interromper o tratamento pode reativar a infecção e piorar a sua doença.
Consulte o seu médico ou farmacêutico antes de começar a usar Hepcludex:
- se o seu fígado não funciona adequadamente – não se sabe em que medida actua Hepcludex nessas circunstâncias; se o seu fígado não funciona bem, não se recomenda usar Hepcludex.
- se teve uma doença renal ou se os análises indicam problemas de rim. Antes e durante o tratamento, o seu médico pode solicitar análises de sangue para comprovar o correcto funcionamento dos rins;
- se padece infecção por VIH ou hepatite C - não se sabe como actua Hepcludex nessas circunstâncias; o seu médico pode solicitar análises de sangue para comprovar o estado da infecção por VIH ou hepatite C
Crianças e adolescentes
As crianças e adolescentes menores de 18 anos de idade não devem ser tratados com Hepcludex.
Outros medicamentos e Hepcludex
Informa ao seu médico se está utilizando, utilizou recentemente ou possa ter que utilizar qualquer outro medicamento.
Alguns medicamentos podem aumentar os efeitos adversos de Hepcludex e não deve tomá-los ao mesmo tempo. Esta é a razão pela qual deve informar o médico se está tomando algum desses medicamentos:
- ciclosporina, um medicamento que inibe o sistema imunológico;
- ezetimiba, utilizado para tratar os níveis elevados de colesterol no sangue;
- irbesartão, utilizado para tratar a hipertensão arterial e as doenças cardíacas;
- ritonavir, utilizado para tratar a infecção por VIH;
- sulfassalazina, utilizada para tratar a artrite reumatoide, a colite ulcerosa e a doença de Crohn.
Alguns medicamentos podem aumentar ou diminuir os efeitos de Hepcludex quando tomados juntos. Em alguns casos, pode ser necessário fazer algumas provas ou o seu médico pode ter que modificar a dose ou submetê-lo a controles periódicos:
- tratamentos contra o cancro (p. ex., dasatinibe, docetaxel, ibrutinibe ou paclitaxel);
- antihistamínicos utilizados para as alergias (p. ex., ebastina ou fexofenadina);
- medicamentos para o sistema imunológico (p. ex., everolimo, sirolimo ou tacrolimo);
- medicamentos para o tratamento da hepatite C e do VIH (p. ex., darunavir, glecaprevir, grazoprevir, indinavir, maraviroc, paritaprevir, saquinavir, simeprevir, tipranavir ou voxilaprevir);
- medicamentos para a diabetes (p. ex., glibenclamida, nateglinida ou repaglinida);
- medicamentos para a disfunção eréctil (p. ex., avanafilo, sildenafilo ou vardenafilo);
- medicamentos para o tratamento da hipertensão arterial e das doenças cardíacas (p. ex., olmesartão, telmisartão ou valsartão);
- estatinas, medicamentos usados para os níveis elevados de colesterol no sangue (p. ex. atorvastatina, fluvastatina, lovastatina, pitavastatina, pravastatina, rosuvastatina ou simvastatina);
- hormonas tiroideias utilizadas para tratar problemas de tiróide;
- alfentanilo, um medicamento opioide que se utiliza para tratar a dor intensa;
- bosentão, utilizado para a hipertensão arterial pulmonar;
- buspirona, um medicamento para a ansiedade;
- budesonida, utilizada para a asma e a doença pulmonar obstructiva crônica;
- conivaptão e tolvaptão, utilizados para tratar a hiponatremia (níveis baixos de sódio);
- darifenacina, utilizada para tratar a incontinência urinária;
- dronedarona, um medicamento para as arritmias cardíacas;
- eletriptano, utilizado para as dores de cabeça do tipo migranoso;
- eplerenona, utilizada para a hipertensão;
- estrona 3-sulfato, um medicamento hormonal para a menopausa;
- felodipino e nisoldipino (medicamentos para o coração);
- lomitapida, utilizada para os níveis elevados de colesterol no sangue;
- lurasidona e quetiapina, medicamentos antipsicóticos para transtornos psiquiátricos;
- midazolam e triazolam, medicamentos para tratar a insónia (incapacidade para dormir) e para anestesia (evitar a dor durante a cirurgia);
- naloxegol, utilizado para tratar a dependência de medicamentos opioides para a dor intensa;
- ticagrelor, anticoagulante para impedir a coagulação do sangue.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento. Não deve usar este medicamento a menos que o seu médico o indique especificamente.
Se é uma mulher em idade fértil, não deve usar este medicamento sem utilizar um método anticonceptivo eficaz.
Fale com o seu médico para decidir se pode dar de mamar durante o tratamento com Hepcludex. Desconhece-se se Hepcludex é excretado no leite materno. Por conseguinte, deve decidir interromper a lactação ou interromper o tratamento com Hepcludex.
Condução e uso de máquinas
Tontura e cansaço são efeitos adversos que podem afetar a capacidade para conduzir e utilizar máquinas. Se tiver alguma dúvida, consulte o seu médico.
Hepcludex contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por ml; isto é, é essencialmente “isento de sódio”.
3. Como usar Hepcludex
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte de novo o seu médico.
Posologia
A dose recomendada é de 2 mg uma vez ao dia em injeção subcutânea (justo debaixo da pele). O seu médico indicará quanto tempo deve usar o medicamento.
O seu médico e enfermeiro ensinarão como preparar e injetar Hepcludex. Este prospecto contém uma guia para a injeção passo a passo para ajudar a injetar o medicamento (ver seção 7).
Se usar mais Hepcludex do que deve
A dose habitual é de 2 mg (1 frasco) ao dia. Se acredita que pode ter recebido mais do que o devido, diga ao seu médico imediatamente.
Se esquecer de usar Hepcludex
Se passaram menos de 4 horas desde que esqueceu a dose de Hepcludex, deve injetar essa dose omitida o mais breve possível e administrar a próxima dose programada à hora habitual.
Se passaram mais de 4 horas desde que esqueceu a dose de Hepcludex, não deve administrar a dose omitida. Deve administrar a próxima dose no dia seguinte à hora habitual. Não deve administrar uma dose dupla para compensar as doses esquecidas. Informe o seu médico se esqueceu de uma dose de Hepcludex.
Se interromper o tratamento com Hepcludex
Se já não deseja continuar a usar Hepcludex, consulte o seu médico antes de interromper o tratamento. Interromper o tratamento pode reativar a infecção e piorar a sua doença. Informe o seu médico imediatamente sobre qualquer mudança nos sintomas após a interrupção do tratamento.
Se tiver alguma outra dúvida sobre o uso de Hepcludex, pergunte ao seu médico ou enfermeiro.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Se experimentar qualquer efeito adverso ou se observar algum não mencionado neste prospecto, consulte o seu médico.
O seguinte efeito adverso é muito frequente(pode afetar mais de 1 de cada 10 pessoas):
- dor de cabeça.
Os seguintes efeitos adversos são frequentes(podem afetar até 1 de cada 10 pessoas):
- tontura
- náuseas
- cansaço
- doença de tipo gripal
- coceira
- dor nas articulações
- reações no local da injeção que podem incluir inchaço, vermelhidão, irritação, hematomas, coceira, erupção cutânea, induração, infecção ou dor local.
Os seguintes efeitos adversos são pouco frequentes(podem afetar até 1 de cada 100 pessoas):
- reações alérgicas, incluindo uma reação anafiláctica (reação alérgica potencialmente mortal repentina).
Os sintomas de reações alérgicas podem incluir:
- falta de ar ou sibilância
- inchaço de face, lábios, língua ou garganta (angioedema)
- erupções cutâneas
- mudanças na tensão arterial ou na frequência cardíaca.
Os sintomas de uma reação anafiláctica são os mesmos que os de uma reação alérgica, mas são mais intensos e requerem assistência médica imediata.
As análises de sangue também podem indicar:
- um aumento de ácidos biliares no sangue (muito frequentes)
- um aumento de glóbulos brancos (eosinófilos) (frequentes).
Comunicação de efeitos adversos
Se experimentar efeitos adversos, consulte o seu médico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para proporcionar mais informação sobre a segurança deste medicamento.
5. Conservação de Hepcludex
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa e no frasco após «CAD». A data de validade é o último dia do mês que se indica.
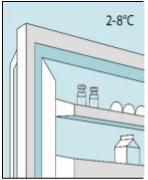
Conservar na geladeira (entre 2 ºC e 8 ºC). Para protegê-los da luz, manter os frascos no envase exterior.
A solução reconstituída deve ser utilizada imediatamente. No entanto, se isso não for possível, pode ser conservada durante um máximo de 2 horas a uma temperatura de até 25 ºC.
Os medicamentos ou as agulhas utilizadas não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e das agulhas utilizadas.
6. Conteúdo do envase e informação adicional
Composição de Hepcludex
O princípio ativo é bulevirtida 2 mg. Cada frasco contém bulevirtida acetato equivalente a 2 mg de bulevirtida.
Os outros componentes são carbonato de sódio anidro, hidrogenocarbonato de sódio, manitol, ácido clorídrico e hidróxido sódico.
Aspecto do produto e conteúdo do envase
Bulevirtida é um pó para solução injetável e apresenta-se sob a forma de pó branco ou esbranquiçado.
Cada caixa contém 30 doses individuais.
Título de autorização de comercialização
Gilead Sciences Ireland UC
Carrigtohill
County Cork, T45 DP77
Irlanda
Responsável pela fabricação
LYOCONTRACT GmbH
Pulverwiese 1
38871 Ilsenburg
Alemanha
ou
Gilead Sciences Ireland UC
IDA Business and Technology Park
Carrigtohill
Co. Cork
Irlanda
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Gilead Sciences Belgium SRL-BV Tel: + 32 (0) 24 01 35 50 | Lituânia Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 |
Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | Luxemburgo Gilead Sciences Belgium SRL-BV Tel: + 32 (0) 24 01 35 50 |
República Checa Gilead Sciences s.r.o. Tel: + 420 (0) 910 871 986 | Hungria Gilead Sciences Ireland UC Tel.: + 353 (0) 1 686 1888 |
Dinamarca Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 | Malta Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Alemanha Gilead Sciences GmbH Tel: + 49 (0) 89 899890-0 | Países Baixos Gilead Sciences Netherlands B.V. Tel: + 31 (0) 20 718 36 98 |
Estônia Gilead Sciences Poland Sp. z o.o. Tel.: +48 (0) 22 262 8702 | Noruega Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 |
Grécia Gilead Sciences Ελλάς Μ.ΕΠΕ. Τηλ: + 30 (0) 210 8930 100 | Áustria Gilead Sciences GesmbH Tel: + 43 (0) 1 260 830 |
Espanha Gilead Sciences, S.L. Tel: + 34 (0) 91 378 98 30 | Polônia Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 |
França Gilead Sciences Tél: + 33 (0) 1 46 09 41 00 | Portugal Gilead Sciences, Lda. Tel: + 351 (0) 21 7928790 |
Croácia Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | Romênia Gilead Sciences (GSR) S.R.L. Tel: +40 31 631 18 00 |
Irlanda Gilead Sciences Ireland UC Tel: + 353 (0) 214 825 999 | Eslovênia Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Islândia Gilead Sciences Sweden AB Sími: + 46 (0) 8 5057 1849 | República Eslovaca Gilead Sciences Slovakia s.r.o. Tel: + 421 (0) 232 121 210 |
Itália Gilead Sciences S.r.l. Tel: + 39 02 439201 | Finlândia Gilead Sciences Sweden AB Puh/Tel: + 46 (0) 8 5057 1849 |
Chipre Gilead Sciences Ελλάς Μ.ΕΠΕ. Τηλ: + 30 (0) 210 8930 100 | Suécia Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Letônia Gilead Sciences Poland Sp. z o.o. Tel.: + 48 (0) 22 262 8702 | Reino Unido (Irlanda do Norte) Gilead Sciences Ireland UC Tel: + 44 (0) 8000 113 700 |
Data da última revisão deste prospecto: <{MM/AAAA}> <{mes AAAA}>.
Este medicamento foi autorizado com uma «autorização condicional». Esta modalidade de autorização significa que se espera obter mais informações sobre este medicamento.
A Agência Europeia de Medicamentos reverá as novas informações sobre este medicamento pelo menos uma vez por ano e este prospecto será atualizado sempre que necessário.
- Guia para a injeção passo a passo
Antes de usar Hepcludex, deve ler primeiro as seções 1-6 deste prospecto.
Antes de iniciar o tratamento com este medicamento em casa, o seu médico ou enfermeiro lhe ensinará como preparar e injetar Hepcludex. Nesta guia, indica-se como deve injetar o medicamento você mesmo. Consulte o seu médico ou enfermeiro se houver algo que não lhe pareça claro, se tiver alguma dúvida ou se precisar de mais informações ou ajuda. Dê-se o tempo necessário para preparar e injetar Hepcludex com cuidado.
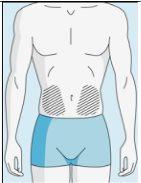
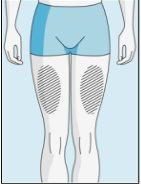
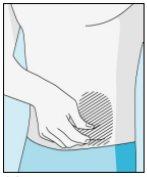
Zonas de injeção | Abdômen | Parte superior do quadril | |
Para reduzir as reações no local da injeção, pode alterar o local da injeção de bulevirtida regularmente. Não injetebulevirtida nas seguintes zonas: joelho, virilha, parte inferior ou interior das nádegas, diretamente em um vaso sanguíneo, ao redor do umbigo, em tecido cicatricial, hematomas, sardas, uma cicatriz cirúrgica, tatuagens ou queimaduras, ou onde tenha ocorrido uma reação na zona de injeção. |
|
| |
|
|
|
|
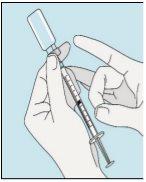
1A Armazenamento | 1B Misturar dose | 1C Lavar as mãos | 1D Limpar o frasco |
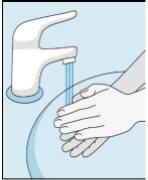
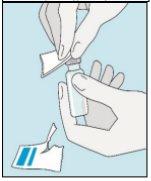
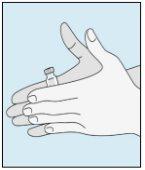
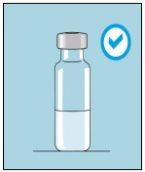
Os frascos de bulevirtida devem ser conservados no embalagem original na geladeira (entre 2 e 8 ºC) para proteger bulevirtida da luz. | Bulevirtida reconstituída deve ser utilizada imediatamente. As seguintes instruções são para dissolver uma dose única. | Lave bem as mãos com sabão e água morna e seque-as com uma toalha limpa. Uma vez que as mãos estejam limpas, não toque em nada mais que o medicamento, o material auxiliar e a zona que rodeia o local da injeção. | Esfregue a parte superior do frasco com um algodão novo embebido em álcool e deixe que seque ao ar. Se tocar na tampa de borracha após limpar, limpe-a novamente com outro algodão embebido em álcool. |
|
|
| |
2A Retirar água estéril | 2B Injetar água estéril no pó | 2C Misturar suavemente bulevirtida | |
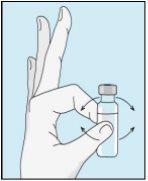
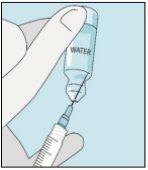
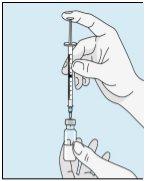
Pegue a seringa. Coloque nela a agulha mais longa. Importante!Certifique-se de que a agulha tampada está bem ajustada, pressionando-a ligeiramente enquanto gira no sentido dos ponteiros do relógio. Retire o capuchão de plástico. Abra a água estéril para preparações injetáveis. Insira a agulha no frasco e inverta suavemente o frasco de água. Certifique-se de que a ponta da agulha esteja sempre abaixo da superfície da água para que não entrem bolhas de ar na seringa. Puxe lentamente o êmbolo até ter 1,0 cc/ml de água estéril dentro da seringa. Retire cuidadosamente a agulha e a seringa do frasco. | Golpeie suavemente o frasco de bulevirtida para que se solte o pó. Insira a agulha com água estéril no frasco de bulevirtida em ângulo. Injete a água estéril lentamente, para que caia pela parede do frasco no pó de bulevirtida. | Golpeie suavemente o frasco de bulevirtida com a ponta dos dedos 10 segundos para que o pó comece a dissolver. Em seguida, gire suavemente o frasco de bulevirtida entre as mãos para que se misture completamente. Certifique-se de que não há pó de bulevirtida grudado nas paredes do frasco. Importante!Não agite o frasco de bulevirtida. Se o agitar, formará espuma e o medicamento demorará muito mais tempo para dissolver. | |
|
| ||
2D Inspecionar bulevirtida | 2E Bulevirtida pronta para a injeção | ||
Quando o pó começar a dissolver, deixe-o e dissolverá completamente. Após dar golpecitos, pode levar até 3 minutos para dissolver. | Quando estiver totalmente misturada, a solução de bulevirtida deve ser transparente. Importante!Bulevirtida totalmente dissolvida deve ser transparente e sem espuma. Se a solução de bulevirtida tiver espuma ou for amarelada, deixe-a dissolver mais tempo. Se vir bolhas, golpeie suavemente o frasco até que desapareçam. Se observar partículas na solução de bulevirtida uma vez dissolvida (por completo), não use esse frasco. Consulte o médico ou farmacêutico que o forneceu. |
|
|
|
|
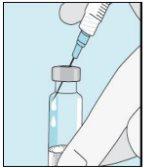
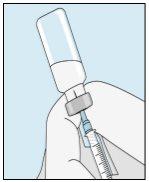
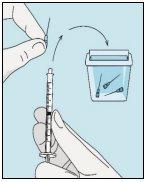
3A Inserir a agulha no frasco | 3B Retirar bulevirtida | 3C Concluir a preparação | 3D Trocar e descartar a agulha |
Pegue a seringa. Insira a agulha no frasco de bulevirtida líquida. | Inverta suavemente o frasco. Certifique-se de que a ponta da agulha esteja sempre abaixo da superfície da solução de bulevirtida para que não entrem bolhas de ar na seringa. Puxe lentamente o êmbolo para introduzir 1,0 cc/ml de bulevirtida. | Golpeie ou sacuda suavemente a seringa e empurre/puxe o êmbolo para eliminar o ar adicional e as bolhas. Para se certificar de terminar com 1,0 cc/ml de bulevirtida na seringa, pode ser necessário puxar o êmbolo além da marca de 1,0 cc/ml. Retire cuidadosamente a agulha e a seringa do frasco. | Retire a agulha mais longa da seringa e descarte-a corretamente para que ninguém se machuque. Importante!Não recoloque o capuchão de plástico na agulha. |
|
|
|
|
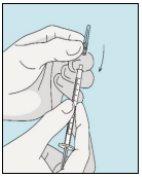
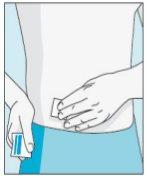
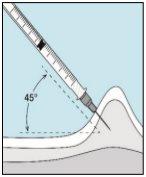
3E Inserir a agulha para a injeção | 3F Escolher o local da injeção | 3G Preparar o local da injeção | 3H Injetar bulevirtida |
Coloque a agulha mais curta na seringa. Importante!Certifique-se de que a agulha tampada está bem ajustada, pressionando-a ligeiramente enquanto gira no sentido dos ponteiros do relógio. Retire o capuchão de plástico. | Escolha um local diferente do que usou para sua última injeção. Limpe o local da injeção com um algodão novo embebido em álcool. Comece no centro, aplique pressão e limpe com um movimento circular para fora. Importante!Deixe secar a zona ao ar. Prepare o frasco de bulevirtida. Limpe novamente a parte superior do frasco de bulevirtida com um algodão novo embebido em álcool. Deixe que seque ao ar. | Pegue um puxão da pele ao redor do local da injeção. | Perfore a pele com um ângulo de 45 graus. Deve-se introduzir a maior parte da agulha. Empurre lentamente o êmbolo todo o caminho para injetar bulevirtida. Retire a agulha da pele. Retire a agulha da seringa e descarte-as corretamente para que ninguém se machuque (ver 3D). |
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVELForma farmacêutica: COMPRIMIDO, 150 mgSubstância ativa: maravirocFabricante: Viiv Healthcare B.V.Requer receita médicaForma farmacêutica: COMPRIMIDO, 300 mgSubstância ativa: maravirocFabricante: Viiv Healthcare B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 90 mgSubstância ativa: enfuvirtideFabricante: Roche Registration GmbhRequer receita médica
Alternativas a HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVEL em Ukraine
Médicos online para HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de HEPCLUDEX 2 mg PÓ PARA SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.