Como usar BRIUMVI 150 MG CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: informação para o paciente
Briumvi 150 mg concentrado para solução para perfusão
ublituximab
Este medicamento está sujeito a acompanhamento adicional, o que agilizará a detecção de nova informação sobre a sua segurança. Pode contribuir comunicando os efeitos adversos que possa ter. A parte final da seção 4 inclui informação sobre como comunicar estes efeitos adversos.
Leia todo o prospecto atentamente antes de começar a tomar este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico.
- Se experimentar efeitos adversos, consulte o seu médico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Briumvi e para que é utilizado
- O que necessita saber antes de começar a receber Briumvi
- Como é administrado Briumvi
- Possíveis efeitos adversos
- Conservação de Briumvi
- Conteúdo do envase e informação adicional
1. O que é Briumvi e para que é utilizado
O que é Briumvi
Briumvi contém o princípio ativo ublituximab. É um tipo de proteína que recebe o nome de anticorpo monoclonal. Os anticorpos actuam unindo-se a alvos específicos do corpo.
Para que é utilizado Briumvi
Briumvi é utilizado para tratar adultos com formas recorrentes de esclerose múltipla (EMR), nas quais o paciente tem surtos (recidivas) seguidos de períodos com sintomas mais suaves ou sem sintomas.
O que é a esclerose múltipla
A esclerose múltipla (EM) afeta o sistema nervoso central, especialmente os nervos do encéfalo e da medula espinhal. Na EM, uns glóbulos brancos denominados linfócitos B, que formam parte do sistema imunitário (o sistema de defesa do corpo), actuam incorrectamente e atacam uma camada protectora (denominada bainha de mielina) que rodeia as células nervosas, causando assim inflamação e lesão. A degradação da bainha de mielina impede que os nervos funcionem corretamente e causa os sintomas da EM. Os sintomas da EM dependem da parte do sistema nervoso central que está afetada e podem incluir problemas ao caminhar e de equilíbrio, fraqueza muscular, formigamento, visão dupla e borrosa, problemas de coordenação e problemas de bexiga.
Nas formas recorrentes da EM, o paciente tem crises repetidas de sintomas (recidivas) que podem aparecer subitamente num prazo de horas ou lentamente ao longo de vários dias. Os sintomas desaparecem ou melhoram entre as recidivas, mas a lesão pode acumular-se e dar lugar a uma deficiência permanente.
Como actua Briumvi?
Briumvi actua unindo-se a um alvo denominado CD20 na superfície dos linfócitos B. Os linfócitos B são um tipo de glóbulo branco que forma parte do sistema imunitário. Na esclerose múltipla, o sistema imunitário ataca a camada protectora que rodeia as células nervosas. Neste processo intervêm os linfócitos B. Briumvi actua sobre os linfócitos B e os elimina e, desta forma, reduz a probabilidade de recidiva, alivia os sintomas e retarda a progressão da doença.
2. O que necessita saber antes de começar a receber Briumvi
Não deve receber Briumvi:
- se é alérgicoao ublituximab ou a algum dos outros componentes deste medicamento (incluídos na seção 6);
- se tem uma infecção grave;
- se lhe disseram que tem problemas graves do sistema imunitário; ou
- se tem cancro.
Se não tem certeza, consulte o seu médico antes de começar a receber Briumvi.
Advertências e precauções
Informar o seu médico antes de começar a receber Briumvise cumpre algum dos critérios indicados a seguir. É possível que o seu médico decida adiar o seu tratamento com Briumvi ou que você não possa receber Briumvi se:
- tem uma infecção. O seu médico esperará até que a infecção seja resolvida antes de lhe administrar Briumvi.
- teve alguma vez hepatite Bou é portador do vírus da hepatite B. Isto deve-se a que medicamentos como Briumvi podem reativar o vírus da hepatite B. Antes do tratamento com Briumvi, o seu médico verificará se você tem risco de infecção pelo vírus da hepatite B. Os pacientes que tiveram hepatite B ou que são portadores do vírus da hepatite B serão submetidos a um exame de sangue e serão objeto de vigilância por um médico por si aparecerem sinais de infecção pelo vírus da hepatite B.
- recebeu recentemente alguma vacina ou poderia receber uma vacina num futuro próximo.
- tem cançoou teve cancro no passado. É possível que o seu médico decida adiar o tratamento.
Reacções relacionadas com a perfusão
- O efeito adverso mais frequente do tratamento com Briumvi são as reacções relacionadas com a perfusão, um tipo de reacções alérgicas que se produzem durante ou pouco depois da administração de um medicamento. Podem ser graves.
- Os sintomas de uma reacção relacionada com a perfusão podem ser, entre outros, os seguintes:
- picazão na pele
- urticária
- vermelhidão da face ou da pele
- irritação da garganta
- problemas para respirar
- inchação da língua ou da garganta
- “pitos” ao respirar (sibilâncias)
- arrepios
- febre
- dor de cabeça
- tontura
- sensação de desmaio
- náuseas
- dor abdominal (no ventre)
- batimento cardíaco rápido
- Informar imediatamente o seu médico ou enfermeiro se tiver ou acreditar que possa ter uma reacção relacionada com a perfusão. As reacções relacionadas com a perfusão podem ocorrer durante a perfusão ou até 24 horas após a perfusão.
- Para reduzir o risco de reacção relacionada com a perfusão, o seu médico lhe administrará outros medicamentos antes de cada perfusão de Briumvi (ver seção 3) e você será objeto de uma vigilância estreita durante a perfusão.
- Se sofrer uma reacção à perfusão, é possível que o seu médico tenha que interromper ou retardar a velocidade da perfusão.
Infecções
- Informar o seu médico antes de receber Briumvi se tiver ou acreditar que possa ter uma infecção. O seu médico esperará até que a infecção seja resolvida antes de lhe administrar Briumvi.
- Poderá contrair infecções com maior facilidade com Briumvi. Isto deve-se a que as células imunitárias sobre as quais actua Briumvi também ajudam a combater as infecções.
- Informar imediatamente o seu médico ou enfermeiro se tiver uma infecção ou algum dos seguintes sinais de infecção durante ou após o tratamento com Briumvi:
- febre ou arrepios
- tosse que não desaparece
- herpes (por exemplo, calenturas [herpes labial], zóster ou úlceras genitais)
- Informar imediatamente o seu médico ou enfermeiro se acreditar que a EM está a piorar ou nota algum sintoma novo.Isto deve-se a uma infecção cerebral muito rara e potencialmente mortal denominada leucoencefalopatia multifocal progressiva (LMP), que pode causar sintomas semelhantes aos da EM. A LMP pode ocorrer em pacientes tratados com medicamentos como Briumvi e outros medicamentos para o tratamento da EM.
- Informar o seu parceiro ou cuidadorsobre o tratamento com Briumvi. Podem notar sintomas da LMP que você não note, como lapsos de memória, problemas de pensamento, dificuldade para caminhar, perda de visão ou mudanças na forma de falar, que o seu médico poderá ter que investigar.
Vacinas
- Informar o seu médico se recebeu recentemente alguma vacina ou poderia receber uma vacina num futuro próximo.
- O seu médico verificará se necessita de alguma vacina antes de começar o tratamento com Briumvi. Deve receber um tipo de vacina chamado vacinas com microorganismos vivos ou com microorganismos vivos atenuados pelo menos 4 semanas antes de começar o tratamento com Briumvi. Durante o tratamento com Briumvi, não deve receber vacinas com microorganismos vivos ou com microorganismos vivos atenuados até que o seu médico lhe indique que o seu sistema imunitário já não está fraco.
- Sempre que seja possível, deve receber outros tipos de vacina chamados vacinas inactivadas pelo menos 2 semanas antes de começar o tratamento com Briumvi. Se desejar receber vacinas inactivadas durante o tratamento com Briumvi, consulte o seu médico.
Crianças e adolescentes
O uso de Briumvi não é indicado em crianças e adolescentes menores de 18 anos. Isto deve-se a que ainda não foi estudado neste grupo etário.
Outros medicamentos e Briumvi
Informar o seu médico ou farmacêutico se está a tomar, tomou recentemente ou poderia ter que tomar qualquer outro medicamento. Em particular, informar o seu médico:
- se está a tomar, tomou recentemente ou poderia ter que tomar medicamentos que afetam o sistema imunitário, como quimioterapia, imunossupressores (excepto corticosteroides) ou outros medicamentos que se utilizam para tratar a EM. Isto deve-se a que podem ter um efeito sumativo sobre o sistema imunitário.
- se tem previsto receber alguma vacina (ver “Advertências e precauções” acima).
Se cumpre algum destes critérios (ou não tem certeza), consulte o seu médico antes de começar a receber Briumvi.
Gravidez e amamentação
- Informar o seu médico antes de começar a receber Briumvi se está grávida, acredita que possa estar grávida ou tem intenção de ficar grávida. Isto deve-se a que Briumvi pode atravessar a placenta e afetar o feto.
- Não utilize Briumvi se está grávida a menos que o tenha discutido com o seu médico. O seu médico sopesará o benefício para si de tomar Briumvi face ao risco para o feto.
- Se tem um filho e recebeu Briumvi durante a gravidez, é importante que comunique ao médico do seu filho que você recebeu Briumvi, para que o médico possa recomendar quando deve ser vacinado o seu filho.
- Desconhece-se se Briumvi passa para o leite materno. Consulte o seu médico a melhor forma de alimentar o seu filho se você receber Briumvi.
Anticoncepção feminina
Se pode ficar grávida (conceber), deve utilizar métodos anticonceptivos:
- durante o tratamento com Briumvi e
- durante pelo menos 4 meses após a última perfusão de Briumvi.
Condução e uso de máquinas
É pouco provável que Briumvi afete a sua capacidade para conduzir ou utilizar máquinas.
Briumvi contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por dose; isto é, é essencialmente “exento de sódio”.
3. Como é administrado Briumvi
Um médico ou um enfermeiro com experiência no uso deste tratamento lhe administrarão Briumvi. Lhe vigiarão estreitamente enquanto lhe estiverem a administrar este medicamento. Isto é por si sofrer algum efeito adverso. Sempre receberá Briumvi com um gotejador (perfusão intravenosa).
Medicamentos que lhe serão administrados antes de receber Briumvi
Antes de receber Briumvi, lhe serão administrados outros medicamentos para prevenir ou reduzir possíveis efeitos adversos tais como reacções relacionadas com a perfusão (ver as seções 2 e 4 para obter informação sobre as reacções relacionadas com a perfusão).
Receberá um corticosteroide e um antihistamínico antes de cada perfusão e é possível que receba também outros medicamentos para reduzir a febre.
Que quantidade de Briumvi receberá e com que frequência a receberá
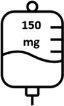
- A primeira dose de Briumvi será de 150 mg. Esta perfusão durará 4 horas.
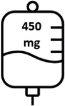
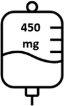
- A segunda dose de Briumvi será de 450 mg administrados 2 semanas após a primeira dose. Esta perfusão durará 1 hora.
- As doses posteriores de Briumvi serão de 450 mg administrados 24 semanas após a primeira dose e cada 24 semanas posteriormente. Estas perfusões durarão 1 hora.
Como é administrado Briumvi
- Um médico ou um enfermeiro lhe administrarão Briumvi. Briumvi deve ser diluído antes da sua administração. Um profissional de saúde realizará a diluição. Será administrado em forma de perfusão numa veia (perfusão intravenosa).
- Será objeto de uma vigilância estreita durante a administração de Briumvi e durante pelo menos 1 hora após a administração das duas primeiras perfusões. Isto é por si sofrer algum efeito adverso tal como uma reacção relacionada com a perfusão. A perfusão pode ser retardada, interrompida temporariamente ou interrompida permanentemente se você sofrer uma reacção relacionada com a perfusão, dependendo da sua gravidade (ver as seções 2 e 4 para obter informação sobre as reacções relacionadas com a perfusão).
Se esquecer uma perfusão de Briumvi
- Se esquecer uma perfusão de Briumvi, consulte o seu médico para programá-la o mais breve possível. Não espere pela próxima perfusão programada.
- Para obter o benefício pleno de Briumvi, é importante que receba cada perfusão no momento oportuno.
Se interromper o tratamento com Briumvi
- É importante que continue o tratamento enquanto você e o seu médico decidam que está a ser benéfico para si.
- Alguns efeitos adversos podem estar relacionados com ter níveis baixos de linfócitos B. Após interromper o tratamento com Briumvi, é possível que experimente estes efeitos adversos até que se restabeleçam os níveis normais de linfócitos B.
- Antes de começar a tomar outros medicamentos, indique ao seu médico quando recebeu a última perfusão de Briumvi.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram.
Foram comunicados os seguintes efeitos adversos com Briumvi:
Efeitos adversos graves
Reacções relacionadas com a perfusão
- As reacções relacionadas com a perfusão são o efeito adverso mais frequente do tratamento com Briumvi (muito frequentes: podem afetar mais de 1 de cada 10 pessoas). Na maioria dos casos são reacções leves, mas podem produzir-se algumas reacções graves.
- Informar imediatamente o seu médico ou enfermeiro se experimentar sinais ou sintomas de uma reacção relacionada com a perfusão durante a perfusão ou até 24 horas após a perfusão.Os sintomas podem ser, entre outros, os seguintes:
- picazão na pele
- urticária
- vermelhidão da face ou da pele
- irritação da garganta
- problemas para respirar
- inchação da língua ou da garganta
- “pitos” ao respirar (sibilâncias)
- arrepios
- febre
- dor de cabeça
- tontura
- sensação de desmaio
- náuseas
- dor abdominal (no ventre)
- batimento cardíaco rápido
- Se experimentar uma reacção relacionada com a perfusão, lhe serão administrados medicamentos para tratar a reacção e é possível que seja necessário retardar ou interromper a perfusão. Quando a reacção tiver cessado, pode ser reanudada a perfusão. Se a reacção relacionada com a perfusão for potencialmente mortal, o seu médico interromperá permanentemente o tratamento com Briumvi.
Infecções
- Poderá contrair infecções com maior facilidade com Briumvi. Algumas delas poderão ser graves. Foram observadas as seguintes infecções em pacientes tratados com Briumvi na EM:
- Muito frequentes(podem afetar mais de 1 de cada 10 pessoas)
- infecções das vias respiratórias altas (infecções de nariz e garganta)
- infecções das vias respiratórias
- Frequentes(podem afetar até 1 de cada 10 pessoas)
- infecções das vias respiratórias baixas (infecções dos pulmões tais como bronquite ou pneumonia)
- infecções herpéticas (calenturas [herpes labial] ou zóster)
- Informar imediatamente o seu médico ou enfermeiro se notar algum dos seguintes sinais de infecção:
- febre ou arrepios
- tosse que não desaparece
- herpes (por exemplo, calenturas [herpes labial], zóster ou úlceras genitais)
Seu médico esperará até que a infecção seja resolvida antes de lhe administrar Briumvi.
Outros efeitos adversos
Frequentes(podem afetar até 1 de cada 10 pessoas)
- neutropenia (nível baixo de neutrófilos, um tipo de glóbulo branco)
- dor em uma extremidade (braços ou pernas)
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informação sobre a segurança deste medicamento.
5. Conservação de Briumvi
Conservar em frigorífico (entre 2 ºC e 8 ºC).
Os profissionais de saúde conservarão Briumvi no hospital ou clínica nas seguintes condições:
- Este medicamento não se deve usar após a data de validade que aparece na caixa e na etiqueta do frasco após “CAD”. A data de validade é o último dia do mês que se indica.
- Este medicamento se deve conservar em frigorífico (entre 2 ºC e 8 ºC). Não se deve congelar. O frasco se deve conservar no embalagem exterior para protegê-lo da luz.
Recomenda-se usar o produto imediatamente após a diluição. Se não for usado imediatamente, os tempos de conservação durante o uso e as condições antes do uso são responsabilidade do profissional de saúde e normalmente não devem exceder 24 horas entre 2 ºC e 8 ºC e posteriormente 8 horas a temperatura ambiente.
Os medicamentos não se devem deitar pelas águas residuais. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Briumvi
- O princípio ativo é ublituximab. Cada frasco contém 150 mg de ublituximab em 6 ml a uma concentração de 25 mg/ml.
- Os demais componentes são cloreto de sódio, citrato de trissódio dihidrato, polissorbato 80, ácido clorídrico e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
- Briumvi é uma solução entre transparente e opalescente e entre incolor e amarelada.
- Apresenta-se sob a forma de concentrado para solução para perfusão.
- Este medicamento está disponível em envases que contêm 1 frasco (frasco de vidro com 6 ml de concentrado).
Título da autorização de comercialização
Neuraxpharm Pharmaceuticals, S.L.
Avda. Barcelona 69
08970 Sant Joan Despí - Barcelona
Espanha
Responsável pela fabricação
Millmount Healthcare
Block 7, City North Business Campus
Stamullen
Co. Meath
Irlanda
K32 YD60
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Neuraxpharm Belgium Tel: +32 (0)2 732 56 95 | Lituânia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Luxemburgo Neuraxpharm France Tel: +32 474 62 24 24 |
República Checa Neuraxpharm Bohemia s.r.o. Tel: +420 739 232 258 | Hungria Neuraxpharm Hungary Kft. Tel: +3630 464 6834 |
Dinamarca Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Suécia) | Malta Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Alemanha neuraxpharm Arzneimittel GmbH Tel: +49 2173 1060 0 | Países Baixos Neuraxpharm Netherlands B.V. Tel: +31 70 208 5211 |
Estônia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Noruega Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Suécia) |
Grécia Brain Therapeutics PC Tel: +302109931458 | Áustria Neuraxpharm Austria GmbH Tel: +43 (0) 2236 320038 |
Espanha Neuraxpharm Spain, S.L.U. Tel: +34 93 475 96 00 | Polônia Neuraxpharm Polska Sp. z.o.o. Tel: +48 783 423 453 |
França Neuraxpharm France Tel: +33 1.53.62.42.90 | Portugal Neuraxpharm Portugal, Unipessoal Lda Tel: +351 910 259 536 |
Croácia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Romênia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Irlanda Neuraxpharm Ireland Ltd Tel: +353 (0)1 428 7777 | Eslovênia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Islândia Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Suécia) | República Eslovaca Neuraxpharm Slovakia a.s. Tel: +421 255 425 562 |
Itália Neuraxpharm Italy S.p.A. Tel: +39 0736 980619 | Finlândia Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Suécia) |
Chipre Brain Therapeutics PC Tel: +302109931458 | Suécia Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 |
Letônia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Reino Unido (Irlanda do Norte) Neuraxpharm Ireland Ltd Tel: +353 (0)1 428 7777 |
Data da última revisão deste prospecto:
Outras fontes de informação
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
Esta informação está destinada apenas a profissionais de saúde:
Leia a ficha técnica ou resumo das características do produto para obter mais informações.
Posologia
- Primeira e segunda dose
A primeira dose é administrada sob a forma de perfusão intravenosa de 150 mg (primeira perfusão), seguida de uma perfusão intravenosa de 450 mg, 2 semanas depois (segunda perfusão).
- Doses posteriores
As doses posteriores de Briumvi são administradas sob a forma de perfusão intravenosa única de 450 mg a cada 24 semanas (tabela 1). A primeira dose posterior de 450 mg deve ser administrada 24 semanas após a primeira perfusão. Deve ser mantido um intervalo mínimo de 5 meses entre cada dose de Briumvi.
Figura 1: Doses e calendário de Briumvi
Primeira perfusão | Segunda perfusão | Perfusões anteriores |
Dia 1
| Dia 15
| A cada 6 meses
|
Tratamento das RRP antes da perfusão
- O tratamento deve ser iniciado e supervisionado por um profissional de saúde com experiência que tenha acesso a um apoio médico adequado para tratar reações graves, tais como reações relacionadas com a perfusão (RRP) graves.
- Pré-medicação para as RRP
Devem ser administrados como pré-medicação os dois medicamentos seguintes antes de cada perfusão de Briumvi para reduzir a frequência e a gravidade das RRP:
- 100 mg de metilprednisolona ou 10-20 mg de dexametasona (ou equivalente) aproximadamente 30-60 minutos antes de cada perfusão de Briumvi;
- difenidramina aproximadamente 30-60 minutos antes de cada perfusão de Briumvi.
Além disso, pode ser considerada também a pré-medicação com um antipirético (p. ex., paracetamol).
Instruções para a diluição
- Briumvi deve ser preparado por um profissional de saúde utilizando uma técnica asséptica. Não agite o frasco.
- O produto está previsto para um único uso.
- Não utilize a solução se apresentar uma mudança de coloração ou contiver partículas sólidas estranhas.
- O medicamento Briumvi deve ser diluído antes da administração. As soluções de Briumvi para administração intravenosa são preparadas mediante diluição do produto em uma bolsa de perfusão que contém cloreto de sódio isotônico a 0,9%. Para a primeira perfusão, dilua um frasco do produto na bolsa de perfusão (150 mg/250 ml) até uma concentração final de aproximadamente 0,6 mg/ml. Para as perfusões posteriores, dilua três frascos do produto na bolsa de perfusão (450 mg/250 ml) até uma concentração final de aproximadamente 1,8 mg/ml.
- Antes de iniciar a perfusão intravenosa, o conteúdo da bolsa de perfusão deve estar à temperatura ambiente.
Forma de administração
- Após a diluição, Briumvi é administrado sob a forma de perfusão intravenosa através de uma via exclusiva.
- As perfusões de Briumvi não devem ser administradas sob a forma de perfusão intravenosa lenta ou em bolo.
Tabela 1: Doses e calendário de Briumvi
Quantidade e volume | Velocidade de perfusão | Duração 1 | |
Primeira perfusão | 150 mg em 250 ml |
2 horas restantes. | 4 horas |
Segunda perfusão (2 semanas depois) | 450 mg em 250 ml |
30 minutos restantes. | 1 hora |
Perfusões posteriores (uma vez a cada 24 semanas) | 450 mg em 250 ml |
30 minutos restantes. | 1 hora |
1A duração da perfusão pode ser maior se a perfusão for interrompida ou retardada.
2A primeira dose posterior deve ser administrada 24 semanas após a primeira perfusão.
Tratamento das RRP durante e após a perfusão
Deve-se vigiar os pacientes durante a perfusão e durante pelo menos uma hora após a finalização das duas primeiras perfusões.
Durante a perfusão
- Ajustes da perfusão em caso de RRP
Em caso de RRP durante uma perfusão, devem ser considerados os seguintes ajustes.
RRP potencialmente mortais
Se houver sinais de uma RRP potencialmente mortal ou incapacitante durante uma perfusão, deve-se suspender imediatamente a perfusão e deve-se administrar ao paciente o tratamento adequado. Nesses pacientes, deve-se interromper de forma permanente o tratamento com Briumvi (ver seção 4.3).
RRP graves
Se um paciente experimentar uma RRP grave, deve-se interromper imediatamente a perfusão e deve-se administrar ao paciente tratamento sintomático. A perfusão só deve ser reiniciada uma vez que todos os sintomas tenham desaparecido. Ao reiniciar a perfusão, comece com a metade da velocidade de perfusão aplicada no momento da ocorrência da RRP. Se a velocidade de perfusão for tolerada, aumente a velocidade como descrito na tabela 1.
RRP de leves a moderadas
Se um paciente experimentar uma RRP de leve a moderada, deve-se reduzir a velocidade de perfusão para a metade da velocidade de perfusão aplicada no momento da ocorrência do evento. Essa velocidade de perfusão reduzida deve ser mantida por pelo menos 30 minutos. Se a velocidade de perfusão reduzida for tolerada, pode-se aumentar a velocidade de perfusão como descrito na tabela 1.
Após a perfusão
- Deve-se observar os pacientes tratados com Briumvi durante pelo menos uma hora após a finalização das duas primeiras perfusões por si aparecerem sintomas de RRP.
- Os médicos devem informar os pacientes de que pode ocorrer uma RRP nas 24 horas seguintes à perfusão.
Período de validade
Frasco não aberto
3 anos
Solução para perfusão intravenosa diluída
- Foi demonstrada a estabilidade química e física durante o uso durante 24 horas entre 2 ºC e 8 ºC e posteriormente durante 8 horas à temperatura ambiente.
- Do ponto de vista microbiológico, a solução para perfusão preparada deve ser usada imediatamente. Se não for usada imediatamente, os tempos de conservação durante o uso e as condições antes do uso são responsabilidade do usuário e normalmente não devem exceder 24 horas entre 2 ºC e 8 ºC e posteriormente 8 horas à temperatura ambiente, a menos que a diluição tenha sido realizada em condições assépticas controladas e validadas.
- Se não for possível finalizar uma perfusão intravenosa no mesmo dia, deve-se descartar a solução restante.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a BRIUMVI 150 MG CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃOForma farmacêutica: PERFURAÇÃO INJETÁVEL, 120 mg (80 mg/kg) belimumabeSubstância ativa: belimumabFabricante: Glaxosmithkline (Ireland) LimitedRequer receita médicaForma farmacêutica: INJETÁVEL, 200 mgSubstância ativa: belimumabFabricante: Glaxosmithkline (Ireland) LimitedRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 400 mg (80 mg/kg) belimumabeSubstância ativa: belimumabFabricante: Glaxosmithkline (Ireland) LimitedRequer receita médica
Médicos online para BRIUMVI 150 MG CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de BRIUMVI 150 MG CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO – sujeita a avaliação médica e regras locais.