BEXSERO Suspensão Injetável em Seringa Pré-carregada

Pergunte a um médico sobre a prescrição de BEXSERO Suspensão Injetável em Seringa Pré-carregada

Como usar BEXSERO Suspensão Injetável em Seringa Pré-carregada
Introdução
Prospecto: informação para o utilizador
Bexserosuspensão injetável em seringa pré-carregada
Vacina meningocócica do grupo B (ADNr, de componentes, adsorvida)
Leia todo o prospecto atentamente antes de si ouseu filho receberem este medicamento, porque contém informações importantes para si ouseu filho.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou enfermeiro.
- Esta vacina foi prescrita apenas para si ou para o seu filho.
- Se experimentar efeitos adversos, consulte o seu médico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto:
- O que é Bexsero e para que se utiliza
- O que precisa saber antes de si ou seu filho receberem Bexsero
- Como usar Bexsero
- Posíveis efeitos adversos
- Conservação de Bexsero
- Conteúdo do envase e informação adicional
1. O que é BEXSERO e para que se utiliza
Bexsero é uma vacina meningocócica do grupo B.
Bexsero contém quatro componentes diferentes da superfície da bactéria Neisseria meningitidisgrupo B.
Bexsero é administrado a indivíduos a partir de 2 meses de idade para ajudá-los a proteger-se contra doenças causadas pelas bactérias Neisseria meningitidisgrupo B. Estas bactérias podem causar infecções graves que, por vezes, podem ser mortais, como a meningite (inflamação da membrana que cobre o cérebro e a medula espinhal) e a sepsis (infecção generalizada do sangue).
A vacina funciona mediante a estimulação específica do sistema natural de defesa do corpo da pessoa vacinada. Isto produz uma proteção contra a doença.
2. O que precisa saber antes de si ou seu filho receberem BEXSERO
NÃO use Bexsero
- Se si ou seu filho são alérgicos aos princípios ativos ou a qualquer um dos outros componentes desta vacina (incluídos na secção 6).
Advertências eprecauções
Consulte o seu médico ou enfermeiro antes de si ou seu filho receberem Bexsero:
- Se si ou seu filho têm uma infecção grave com febre alta. Nesse caso, a vacinação será adiada. A presença de uma infecção menor, como um resfriado, não é motivo para adiar a vacinação, mas consulte primeiro o seu médico ou enfermeiro.
- Se si ou seu filho têm hemofilia ou outros problemas que possam afetar a coagulação do sangue, como um tratamento com anticoagulantes. Consulte primeiro o seu médico ou enfermeiro.
- Se si ou seu filho recebem tratamento que bloqueia a parte do sistema imunológico conhecida como ativação do complemento, como eculizumab. Mesmo que si ou seu filho tenham sido vacinados com Bexsero, si ou seu filho continuam tendo um maior risco de doença invasiva causada pela bactéria Neisseria meningitidisgrupo B.
- Se seu filho nasceu prematuramente (a las 28 semanas de gestação ou antes), especialmente se teve dificuldades respiratórias. Deixar de respirar ou respirar de forma irregular durante um breve período de tempo pode ser mais comum nos três primeiros dias após a vacinação destes bebês e pode requerer monitorização especial.
- Se si ou seu filho têm alergia ao antibiótico kanamicina. Se existe, o nível de kanamicina na vacina é baixo. Se for possível que si ou seu filho tenham uma alergia à kanamicina, consulte primeiro o seu médico ou enfermeiro.
Pode produzir-se desfalecimento, sensação de perda de consciência ou outras reações associadas ao estresse como resposta a qualquer injeção com agulha. Informe o seu médico ou enfermeiro se teve uma reação deste tipo no passado.
Não existem dados sobre o uso de Bexsero em adultos maiores de 50 anos. Os dados sobre o uso de Bexsero em pacientes com condições médicas crónicas ou com o sistema imunológico débil são limitados. Se si ou seu filho têm o sistema imunológico débil (por exemplo, devido a fármacos imunossupressores, infecção por VIH ou defeitos congénitos do sistema natural de defesa do corpo), é possível que a eficácia de Bexsero seja reduzida.
Como qualquer vacina, Bexsero pode não proteger completamente a todas as pessoas vacinadas.
Uso de Bexsero com outros medicamentos
Informe o seu médico ou enfermeiro se si ou seu filho estão tomando, tomaram recentemente ou podem ter que tomar qualquer outro medicamento ou receberam recentemente alguma outra vacina.
Bexsero pode ser administrado ao mesmo tempo que qualquer um dos seguintes componentes de vacina: difteria, tétano, tosse ferina (pertussis), Haemophilus influenzaetipo b, poliomielite, hepatite B, neumococo, sarampo, papeira, rubéola, varicela e meningocócicos A, C, W, Y. Para mais informações, consulte o seu médico ou enfermeiro.
Quando é administrado simultaneamente com outras vacinas, Bexsero deve ser administrado em zonas de inyeção independentes.
O médico ou enfermeiro pode pedir-lhe que administre ao seu filho fármacos que reduzam a febre no momento de administrar Bexsero e depois. Isto ajudará a reduzir os efeitos adversos de Bexsero.
Gravidez elactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de ser administrado Bexsero. Pode ser que o seu médico continue recomendando a administração de Bexsero se tiver risco de exposição a uma infecção meningocócica.
Condução euso de máquinas
A influência de Bexsero sobre a capacidade para conduzir e utilizar máquinas é nula ou insignificante. No entanto, algum dos efeitos mencionados na secção 4 “Posíveis efeitos adversos” pode afetar de forma temporária a capacidade para conduzir ou utilizar máquinas.
Bexsero contém cloreto de sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por dose; isto é, essencialmente “isento de sódio”.
3. Como usar BEXSERO
Bexsero (0,5 ml) será administrado a si ou ao seu filho por um médico ou enfermeiro. Será injetado num músculo, normalmente na coxa em lactentes ou no braço superior em crianças, adolescentes e adultos.
É importante que siga as instruções do médico ou enfermeiro para completar a série de injeções.
Lactentes desde 2 meses até5meses de idade no momento da primeira dose
Seu filho deve receber uma série inicial de duas ou três injeções da vacina seguida de uma injeção adicional (dose de recorde).
- A primeira injeção não deve ser administrada antes dos 2 meses de idade.
- Se são administradas três doses iniciais, o intervalo entre injeções deve ser de, pelo menos, 1 mês.
- Se são administradas duas doses iniciais, o intervalo entre injeções deve ser de, pelo menos, 2 meses.
- Uma dose de recorde será administrada entre os 12 e os 15 meses de idade após um intervalo de, pelo menos, 6 meses desde a última injeção da série inicial. Em caso de atraso na administração, a dose de recorde não deve ser administrada mais tarde dos 24 meses de idade.
Lactentes de6a11meses deidade no momento da primeira dose
Os lactentes de entre 6 e 11 meses de idade devem receber duas injeções da vacina seguidas de uma injeção adicional (dose de recorde).
- O intervalo entre cada injeção deve ser de, pelo menos, 2 meses.
- Uma dose de recorde será administrada no segundo ano de vida, após um intervalo de, pelo menos, 2 meses desde a segunda injeção.
Crianças de12meses a23meses de idade no momento da primeira dose
As crianças de 12 a 23 meses de idade devem receber duas injeções da vacina, seguidas de uma injeção adicional (dose de recorde).
- O intervalo entre cada injeção deve ser de, pelo menos, 2 meses.
- Uma dose de recorde será administrada após um intervalo de 12 a 23 meses desde a segunda injeção.
Crianças de2a10anos de idade no momento da primeira dose
As crianças de 2 a 10 anos de idade devem receber duas injeções da vacina.
- O intervalo entre cada injeção deve ser de, pelo menos, 1 mês.
- Seu filho pode receber uma injeção adicional (recorde).
Adolescentes eadultos desde os 11 anos de idade no momento da primeira dose
Os adolescentes (desde 11 anos de idade) e os adultos devem receber duas injeções da vacina.
- O intervalo entre cada injeção deve ser, pelo menos, 1 mês.
- Si pode receber uma injeção adicional (recorde).
Adultos maiores de50anos
Não há dados sobre os adultos maiores de 50 anos. Pergunte ao seu médico se seria benéfica para si a administração de Bexsero.
Se tiver alguma outra dúvida sobre Bexsero, pergunte ao seu médico ou enfermeiro.
4. Posíveis efeitos adversos
Como todas as vacinas, esta vacina pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Quando Bexsero é administrado a si ou ao seu filho, os efeitos adversos muito frequentes (podem afetar a mais de 1 de cada 10 pessoas) que si ou seu filho podem ter são (observados em todos os grupos de idade):
- Dor/dor aguda à pressão no local da injeção, vermelhidão da pele no local da injeção, inchaço da pele no local da injeção, endurecimento da pele no local da injeção.
Após receber esta vacina, podem produzir-se também os seguintes efeitos adversos.
Lactentes ecrianças (até10anos de idade)
Muito frequentes(podem afetar a mais de 1 de cada 10 pessoas): febre (≥ 38 °C), perda do apetite, dor aguda à pressão no local de injeção (incluindo dor intensa no local de injeção que provoca choro ao mover a extremidade na qual foi administrada a injeção), articulações dolorosas, erupção cutânea (crianças de 12 a 23 meses de idade) (pouco frequente após a dose de recorde), sonolência, irritabilidade, choro incomum, vómitos (pouco frequente após a dose de recorde), diarreia, dor de cabeça.
Frequentes(podem afetar até 1 de cada 10 pessoas): erupção cutânea (lactentes e crianças de 2 a 10 anos de idade)
Pouco frequentes(podem afetar até 1 de cada 100 pessoas): febre alta (≥40 °C), convulsões (incluindo convulsões febris), pele seca, palidez (rara após a dose de recorde)
Raros(podem afetar até 1 de cada 1.000 pessoas): doença de Kawasaki, que pode incluir sintomas como febre que dura mais de cinco dias, associada a erupção cutânea no tronco e, por vezes, seguida de descamação da pele das mãos e dedos, inchaço glandular no pescoço e vermelhidão dos olhos, lábios, garganta e língua. Erupção com picor, erupção cutânea
Adolescentes (desde11anos de idade) eadultos
Muito frequentes(podem afetar a mais de 1 de cada 10 pessoas): dor no local da injeção que impede realizar a atividade diária normal, dor em músculos e articulações, náuseas, indisposição geral, dor de cabeça.
Os efeitos adversos notificados durante o uso comercial são:
Gânglios linfáticos aumentados.
Reações alérgicas, que podem incluir inchaço intenso dos lábios, da boca, da garganta (que pode provocar dificuldade para engolir), dificuldade para respirar com sibilância (silvos ao respirar) ou tosse, erupção, perda de consciência e pressão arterial muito baixa.
Colapso (aparição de flacidez muscular repentina), nível de resposta menor do que o normal ou perda de consciência e palidez ou coloração azulada da pele nos crianças pequenas.
Sensação de perda de consciência ou desfalecimento.
Erupção cutânea (adolescentes desde os 11 anos de idade e adultos).
Febre (adolescentes desde os 11 anos de idade e adultos).
Reações no local da injeção como inchaço extenso da extremidade vacinada, bolhas no local da injeção ou na área que a rodeia e bulto duro no local da injeção (que pode durar mais de um mês). De forma esporádica, foram notificados, pouco após a vacinação, rigidez de nuca ou excessiva sensibilidade à luz (fotofobia) indicativos de irritação meníngea; estes sintomas foram de natureza leve e transitória.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, si pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de BEXSERO
Mantenha esta vacina fora da vista e do alcance das crianças.
Não utilize esta vacina após a data de validade que aparece na caixa e na etiqueta da seringa pré-carregada após CAD. A data de validade é o último dia do mês que se indica.
Conservar em frigorífico (entre 2 ?C e 8 ?C). Não congelar.
Conservar no embalagem original para protegê-lo da luz.
Os medicamentos não devem ser jogados nos deságues nem na lixeira. Pergunte ao seu médico ou enfermeiro como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Bexsero
Uma dose (0,5 ml) contém:
Princípios ativos
Proteína1,2,3 recombinante de fusão NHBA de Neisseria meningitidisdo grupo B | 50 microgramas |
Proteína1,2,3 recombinante NadA de Neisseria meningitidisdo grupo B | 50 microgramas |
Proteína1,2,3 recombinante de fusão fHbp de Neisseria meningitidisdo grupo B | 50 microgramas |
Vesículas da membrana externa (OMV) de Neisseria meningitidisgrupo B cepa NZ98/254 medida como a quantidade total de proteína que contém o PorA P1.4 | 25 microgramas |
1 produzida em células de E. colicom a tecnologia de ADN recombinante.
2 adsorvida em hidróxido de alumínio (0,5 mg Al3+).
3 NHBA (antígeno de Neisseriade união a heparina), NadA (adesina A de Neisseria), fHbp (proteína de união ao fator H)
Os demais componentes:
Cloruro de sódio, histidina, sacarose e água para preparações injetáveis (ver seção 2 para mais informações sobre o sódio).
Aspecto do produto econteúdo do envase
Bexsero é uma suspensão branca opalescente.
Bexsero está disponível em seringa precarregada de 1 dose com ou sem agulhas separadas; tamanhos de envase de 1 e 10.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização:
GSK Vaccines S.r.l.
Via Fiorentina 153100 SienaItália.
Responsável pela fabricação:
GSK Vaccines S.r.l.
Bellaria‑Rosia53018 Sovicille (Siena)Itália.
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Bélgica GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 | Lituânia GSK Vaccines S.r.l. Tel: +370 80000334 |
Bulgária GSK Vaccines S.r.l. Tel: +359 80018205 | Luxemburgo GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 |
República Tcheca GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Hungria GSK Vaccines S.r.l. Tel.: +36 80088309 |
Dinamarca GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GSK Vaccines S.r.l. Tel: +356 80065004 |
Alemanha GlaxoSmithKline GmbH & Co. KG Tel: +49 (0)89 36044 8701 | Países Baixos GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Estônia GSK Vaccines S.r.l. Tel: +372 8002640 | Noruega GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Grécia GlaxoSmithKline Μονοπρ?σωπη A.E.B.E Tηλ: + 30 210 68 82 100 | Áustria GlaxoSmithKline Pharma GmbH. Tel: + 43 (0)1 97075 0 |
Espanha GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polônia GSK Services Sp. z o.o. Tel.: + 48 (22) 576 9000 |
França Laboratoire GlaxoSmithKline Tél: + 33 (0) 1 39 17 84 44 | Portugal GlaxoSmithKline ‑ Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Croácia GSK Vaccines S.r.l. Tel.: +385 800787089 | Romênia GSK Vaccines S.r.l. Tel: +40 800672524 |
Irlanda GlaxoSmithKline (Irlanda) Ltd Tel: + 353 (0)1 495 5000 | Eslovênia GSK Vaccines S.r.l. Tel: +386 80688869 |
Islândia Vistor hf. Sími: +354 535 7000 | República Eslovaca GSK Vaccines S.r.l. Tel: +421 800500589 |
Itália GlaxoSmithKline S.p.A. Tel: +39 (0)45 7741 111 | Finlândia GlaxoSmithKline Oy Puh/Tel: + 358 10 30 30 30 |
Chipre GSK Vaccines S.r.l. Τηλ: +357 80070017 | Suécia GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Letônia GSK Vaccines S.r.l. Tel: +371 80205045 | Reino Unido (Irlanda do Norte) GSK Vaccines S.r.l. Tel: + 44(0) 800 221441 |
Data da última revisão desteprospeto:
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
Esta informação é destinada apenasaprofissionais do setor sanitário:
Durante o armazenamento pode ser observado um depósito fino branco na suspensão da seringa precarregada.
Agite bem a vacina antes de usá-la para formar uma suspensão homogênea.
A vacina deve ser inspecionada visualmente para verificar se há partículas ou descoloração antes da administração.
Em caso de que sejam observadas partículas estranhas e/ou alteração do aspecto físico, não administre a vacina. Se o envase contém duas agulhas de diferentes comprimentos, escolha a mais adequada para garantir que a vacina possa ser administrada por via intramuscular.
Não congelar.
Bexsero não deve ser misturado com outras vacinas na mesma seringa.
Se for necessário administrá-lo de forma simultânea com outras vacinas, deve ser feito em zonas de injeção separadas.
Deve ser garantido que a vacina seja injetada por via intramuscular apenas.
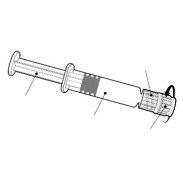
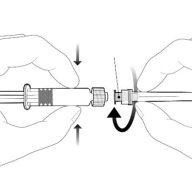
Instruções para a seringa precarregada
| Segure a seringa pelo corpo, não pelo êmbolo. Desenrosque a tampa da seringa girando-a no sentido contrário ao das agulhas do relógio. |
| Para inserir a agulha, conecte a base ao adaptador luer-locke gire-o um quarto de volta no sentido das agulhas do relógio até que sinta que se bloqueia. Não retire o êmbolo da seringa do corpo. Se isso ocorrer, não administre a vacina. |
Eliminação de resíduos
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a regulamentação local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a BEXSERO Suspensão Injetável em Seringa Pré-carregadaForma farmacêutica: INJETÁVEL, 0,5 ml dose únicaSubstância ativa: meningococcus B, multicomponent vaccineFabricante: Glaxosmithkline Vaccines S.R.L.Requer receita médicaForma farmacêutica: INJETÁVEL, 120 microgramas + 120 microgramasSubstância ativa: meningococcus B, multicomponent vaccineFabricante: Pfizer Europe Ma EeigRequer receita médicaForma farmacêutica: INJETÁVEL, 0,5 mlFabricante: Sanofi Winthrop IndustrieRequer receita médica
Alternativas a BEXSERO Suspensão Injetável em Seringa Pré-carregada noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a BEXSERO Suspensão Injetável em Seringa Pré-carregada em Ukraine
Médicos online para BEXSERO Suspensão Injetável em Seringa Pré-carregada
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de BEXSERO Suspensão Injetável em Seringa Pré-carregada – sujeita a avaliação médica e regras locais.