ZTALMY 50 mg/mL ORAL SUSPENSION

How to use ZTALMY 50 mg/mL ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
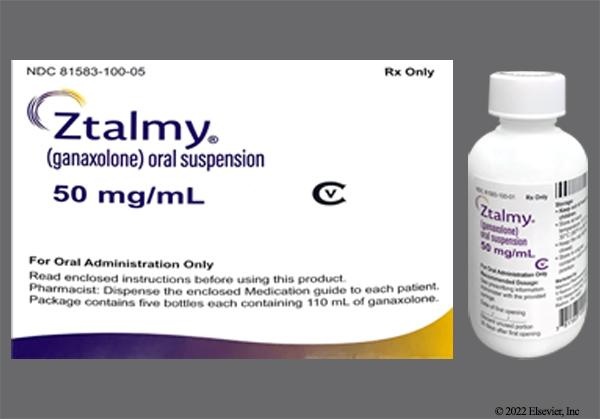
ZTALMY 50 mg/ml Oral Suspension
ganaxolone
This medication is subject to additional monitoring, which will make it easier to detect new information about its safety. You can contribute by reporting any side effects you or your child may experience. The last part of section 4 contains information on how to report side effects.
Read all of this leaflet carefully before you or your child start taking this medicine, because it contains important information.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you or your child only. Do not give it to others, as it may harm them, even if they have the same symptoms as you or your child.
- If you experience side effects, contact your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is ZTALMY and what is it used for
- What you or your child need to know before taking ZTALMY
- How to take ZTALMY
- Possible side effects
- Storage of ZTALMY
- Package Contents and Further Information
1. What is ZTALMY and what is it used for
ZTALMY contains the active substance ganaxolone, a neuroactive steroid that acts by binding to specific receptors and preventing the brain from having epileptic seizures.
ZTALMY is used to treat a rare disorder that causes epileptic seizures called "cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder" in patients aged 2 to 17 years.
If ZTALMY is helpful in treating epileptic seizures, it can be continued when you or your child turn 18 years old.
ZTALMY is used in combination with other anti-epileptic medications.
This medication will reduce the number of daily epileptic seizures you or your child may have.
2. What you or your child need to know before taking ZTALMY
Do not take ZTALMYif you are allergic to ganaxolone or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
Consult your doctor or pharmacist before starting to take ZTALMY:
ZTALMY may cause drowsiness or a tendency to sleep, or a feeling of being too calm and relaxed (i.e., a sedated feeling). Consult your doctor or pharmacist before starting to take ZTALMY if you have any doubts about these effects or if you are taking central nervous system depressants, such as other medications for treating epileptic seizures, opioids, antidepressants, or alcohol, as they may increase drowsiness and the sedative effects of ZTALMY.
If you notice unusual changes in your mood or behavior or if you think about self-harm or suicide, contact your doctor immediately.
If you are in charge of a child with CDKL5 deficiency disorder, be aware of any unusual changes in their mood or behavior or anything they say that may indicate they are thinking about self-harm or suicide. If you observe any of these signs, contact your doctor immediately.
ZTALMY may be abused or misused. Consult your doctor or pharmacist before starting to take ZTALMY if you have a medical history of alcohol or drug abuse.
Your doctor will closely monitor you during treatment and may reduce the dose of ZTALMY.
Children and Adolescents
ZTALMY should not be given to children under 2 years of age, as there is no information available on its use in children under this age.
Other Medicines and ZTALMY
Tell your doctor or pharmacist if you or your child are taking, have recently taken, or might take any other medicines. Taking ZTALMY with other medicines may cause side effects or affect how well other medicines or ZTALMY work.
Do not start taking other medicines or stop taking them without consulting your doctor or pharmacist first.
Tell your doctor if you or your child are taking any of the following medicines, as the dose of ZTALMY may need to be adjusted:
- Medicines containing valproate, used to treat epilepsy, as they may require a lower dose of ZTALMY;
Medicines that may reduce the effect of ZTALMY may require a higher dose of ZTALMY:
- Other anti-epileptic or anti-convulsant medicines such as carbamazepine, phenytoin, phenobarbital, and primidone;
- Antibiotics such as rifampicin;
- St. John's Wort (Hypericum perforatum), a herbal remedy used for mild depression.
No interaction has been investigated between this medicine and oral contraceptives. Tell your doctor if you are taking oral contraceptives.
Taking ZTALMY with Alcohol
Do not consume alcohol, as it may increase drowsiness and the sedative effects of ZTALMY.
Pregnancy
If you are pregnant or think you may be pregnant, consult your doctor or pharmacist before using this medicine.
ZTALMY should not be taken during pregnancy or in women of childbearing age who are not using an effective contraceptive method.
Breast-feeding
Do not use ZTALMY during breast-feeding unless your doctor decides that the benefits of taking ZTALMY outweigh the possible risks.
Driving and Using Machines
ZTALMY may make you feel drowsy. If you are affected, do not drive vehicles, ride bicycles, or use machines until you feel more alert.
ZTALMY contains Sodium
This medicine contains less than 1 mmol of sodium (23 mg) per ml, i.e., it is essentially
"sodium-free".
ZTALMY contains Sodium Benzoate and Benzoic Acid
This medicine contains 0.92 mg of sodium benzoate and 0.00068 mg of benzoic acid in each ml. Sodium benzoate and benzoic acid may increase jaundice (yellowing of the skin and eyes) in newborns (up to 4 weeks of age).
ZTALMY contains Benzyl Alcohol
This medicine contains 0.00023 mg of benzyl alcohol in each ml. Benzyl alcohol may cause allergic reactions. Benzyl alcohol has been linked to the risk of serious side effects, such as respiratory problems (called "gasping syndrome") in small children. It should not be given to your newborn baby (up to 4 weeks of age), unless advised by your doctor. Do not use it for more than one week in small children (under 3 years of age), unless advised by your doctor or pharmacist. Increased risk due to accumulation in small children. Consult your doctor or pharmacist if you are pregnant or breast-feeding or if you have liver or kidney disease. The reason is that large amounts of benzyl alcohol can accumulate in your body and cause side effects (metabolic acidosis).
ZTALMY contains Methyl Parahydroxybenzoate and Propyl Parahydroxybenzoate
This medicine contains 1.02 mg of methyl parahydroxybenzoate and 0.2 mg of propyl parahydroxybenzoate in each ml, which may cause allergic reactions (possibly delayed).
3. How to Take ZTALMY
ZTALMY is administered under the supervision of a doctor experienced in the treatment of epilepsy. Follow the administration instructions for this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
This is an oral suspension (a liquid to be swallowed). Your doctor or pharmacist will tell you the amount (in ml) of oral suspension to take each day and how many times a day to take it.
Your doctor will calculate the dose based on your body weight. You may start with a low dose that your doctor will gradually increase over time.
If you have severe liver failure, your doctor will initially prescribe a lower dose and follow a different dose adjustment program.
Patient with a body weight less than or equal to28 kg
You or your child will have the dose gradually increased over 4 weeks until you receive the maximum recommended daily dose of 63 mg/kg/day administered in three separate doses every 8 hours.
Patient with a body weight greater than28 kg
You or your child will have the dose gradually increased over 4 weeks until you receive the maximum recommended daily dose of 1800 mg/day administered in three separate doses every 8 hours.
It is recommended to take 3 equal doses throughout the day. However, ZTALMY may cause drowsiness, so your doctor may decide to administer a lower dose during the day and a higher dose at night to avoid making you feel drowsy during the day.
Consult your doctor if you are unsure about the dose or if you think it may need to be changed.
How to take ZTALMY
- Take the medicine with meals or shortly after.
- If possible, try to take it with similar types of food (e.g., with a similar fat content) to get the same effect each time.
- Do not mix ZTALMY with food or drinks.
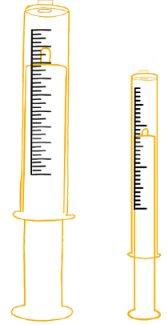
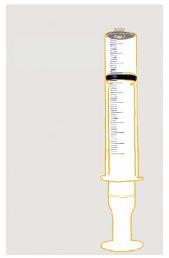
- To ensure an exact dose, use the reusable oral dosing syringes provided with each package.
Instructions for use
Each single-bottle package is supplied with:
A bottle of oral suspension closed with a child-resistant cap |
|
Two reusable oral dosing syringes of 12 ml and two of 3 ml. |
|
A bottle adapter |
|
ZTALMY is also supplied in a package with five bottles of oral suspension, five reusable oral dosing syringes of 12 ml, and five bottle adapters. Note that the package containing five bottles of ZTALMY does not include reusable oral dosing syringes of 3 ml.
- Ask your doctor, pharmacist, or nurse if you are unsure how to prepare or take the prescribed dose of ZTALMY.
- You will find reusable oral dosing syringes of 12 ml and 3 ml in the single-bottle package. If your dose is 3 ml or less, use the smaller 3 ml syringes to take the medicine. If your dose is more than 3 ml, use the larger 12 ml syringes to take the dose.
- Always use the appropriate reusable oral dosing syringe provided with ZTALMY to ensure you measure the correct amount of ZTALMY. Do not use a kitchen spoon. Do not mix ZTALMY with food or drinks to administer it.
- Each 3 ml dosing syringe can be used for 16 consecutive days. After 16 days, discard the used dosing syringe and use the spare syringe contained in the box.
- Use ZTALMY within 30 days of opening the bottle. There is space on the bottle label to note the date when the bottle should be discarded after opening, so you do not forget.
- After 30 days, discard any unused ZTALMY and use a new bottle.
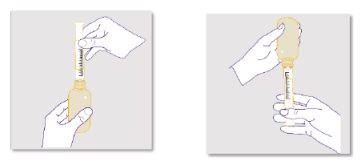
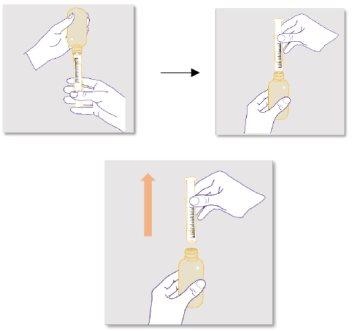
Preparing the bottle:
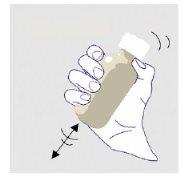
Always shake the bottle well for 1 minute and then let it sit for another minute to allow the foam that forms during shaking to settle before measuring and administering each doseof ZTALMY. This will help you measure the correct amount of medicine. NOTE; this step is necessary for each intake of the medicine. |
|
|
|
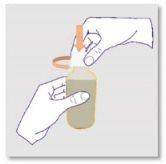
NOTE: this step is only necessary the first time you use the bottle |
|
NOTE: once inserted, do notremove the pressure adapter from the bottle. |
|
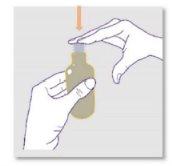
Preparing the dose:
It is important to use the correct reusable oral dosing syringe to measure your dose:
|
|
If there is an air bubble inside the syringe, return the liquid to the bottle while keeping it upside down and repeat step 6 until the air bubble is removed. |
|
|
|
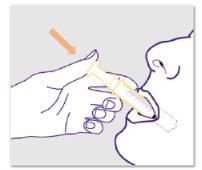
Taking or administering ZTALMY:
|
|
|
|
Warning: Do not use bleach or any other strong cleaning solution. Do not wash the oral syringe in the dishwasher. |
|
When using the 12 ml syringe that comes with each bottle, do not discard the reusable oral syringes until the bottle is empty. When using the 3 ml syringe, discard it after 16 days. |
|
|
If you take more ZTALMY than you should
If you accidentally take more ZTALMY than you should, inform your doctor or pharmacist immediately, or contact the emergency department of the nearest hospital, and take the medicine with you. You may feel drowsy or sleepy if you take too much medicine.
If you forget to take ZTALMY
If you forget to take a dose, the missed dose can be taken until 4 hours before the next scheduled dose. If there are less than 4 hours left for the next dose, it is recommended to skip the missed dose and continue with the next scheduled dose.
If you stop taking ZTALMY
Do not reduce the dose or stop taking ZTALMY without consulting your doctor first. Stopping this treatment abruptly may increase your epileptic seizures. Your doctor will explain how to gradually stop treatment with ZTALMY.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
You may have the following adverse effects with this medicine. Tell your doctorif you experience any of the following adverse effects:
Very Common(may affect more than 1 in 10 people):
- drowsiness;
- fever.
Common(may affect more than 1 in 100 people):
- feeling of excessive calmness or relaxation;
- feeling of great tiredness during the day or sleeping more than usual at night;
- lack of energy:
- drooling;
- producing more saliva than normal.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is an adverse effect that does not appear in this leaflet. You can also report them directly through the national reporting system included in Annex V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of ZTALMY
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and on the label of the bottle after CAD. The expiration date is the last day of the month indicated.
This medicine does not require any special storage temperature. Discard any unused medicine 30 days after opening the bottle for the first time.
Medicines should not be thrown down the drain or into the trash. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
ZTALMY Composition
- The active ingredient is ganaxolone. Each ml of oral suspension contains 50 mg of ganaxolone.
- The other components are: hypromellose (E464), polyvinyl alcohol (E1203), sodium lauryl sulfate (E487), methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), sodium benzoate (E211), anhydrous citric acid (E330), sodium citrate dihydrate (E311), artificial cherry flavor (including propylene glycol [E1520] and benzyl alcohol [E1519]), sucralose (E955), simethicone emulsion (simethicone, polysorbate 65, methylcellulose, polyethylene, glycol monoester, glycerol monoester, xanthan gum, benzoic acid [E210], sorbic acid, and purified water), purified water (see also in section 2 "ZTALMY contains sodium", "ZTALMY contains sodium benzoate", "ZTALMY contains benzoic acid", "ZTALMY contains benzyl alcohol" and "ZTALMY contains methyl parahydroxybenzoate and propyl parahydroxybenzoate").
Product Appearance and Package Contents
ZTALMY is a white to off-white oral suspension. It is presented in a plastic bottle with a child-resistant plastic cap. Each bottle contains 110 ml of oral suspension.
ZTALMY is presented in packages of:
- one bottle of oral suspension, two 12 ml oral dosing syringes, and two 3 ml oral dosing syringes, and a bottle adapter; or
- five bottles of oral suspension, five 12 ml oral dosing syringes, and five bottle adapters.
Only some package sizes may be marketed.
Marketing Authorization Holder
Marinus Pharmaceuticals Emerald Limited
10 Earlsfort Terrace
Dublin 2
D02 T380
Ireland
Manufacturer
Orion Corporation Orion Pharma
Joensuunkatu 7
FI-24100 Salo
Finland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Luxembourg/Luxemburg Orion Pharma BVBA/SPRL Tel: +32 (0)15 64 10 20 | Danmark Orion Pharma A/S Tlf: +45 8614 00 00 |
Nederland Orion Pharma BVBA/SPRL Tel: +32 (0)15 64 10 20 | Eesti Orion Pharma Eesti Oü Tel: +372 6 644 550 |
Ceská republika Orion Pharma s.r.o. Tel: +420 234 703 305 | España Orion Pharma S.L. Tel: + 34 91 599 86 01 |
Deutschland Österreich Orion Pharma GmbH Tel: + 49 40 899 6890 | Ireland Orion Pharma (Ireland) Ltd. c/o Allphar Services Ltd. Tel: + 353 1 428 7777 |
Ελλáδα Orion Pharma Hellas M.E.Π.E Τηλ: + 30 210 980 3355 | Italia Orion Pharma S.r.l. Tel: + 39 02 67876111 |
France Orion Pharma Tél: +33 (0) 1 85 18 00 00 | Latvija Orion Corporation Orion Pharma parstavnieciba Tel: +371 20028332 |
Ísland Vistor hf. Simi: +354 535 7000 | Magyarország Orion Pharma Kft. Tel.: +36 1 239 9095 |
Κúπρος Lifepharma (ZAM) Ltd Τηλ.: +357 22056300 | Polska Orion Pharma Poland Sp. z.o.o. Tel.: + 48 22 8 333 177 |
Lietuva UAB Orion Pharma Tel: +370 5 276 9499 | Slovenija Orion Pharma d.o.o. Tel: +386 (0) 1 600 8015 |
Norge Orion Pharma AS Tlf: + 47 4000 4210 | Suomi/Finland Orion Corporation Puh/Tel: + 358 10 4261 |
Portugal Orionfin Unipessoal Lda Tel: + 351 21 154 68 20 | România Orion Corporation Tel: + 358 10 4261 |
Hrvatska Orion Pharma d.o.o. Tel: +386 (0) 1 600 8015 | United Kingdom (Northern Ireland): Orion Pharma (Ireland) Ltd. c/o Allphar Services Ltd. Tel: +353 1 428 7777 |
Malta Salomone Pharma Tel: +356 21220174 | Sverige Orion Pharma AB Tel: + 46 8 623 6440 |
Slovenská republika Orion Pharma s.r.o. Tel: +420 234 703 305 | |
|
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZTALMY 50 mg/mL ORAL SUSPENSIONDosage form: TABLET, 100 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 200 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: TABLET, 25 mgActive substance: topiramateManufacturer: Adamed Laboratorios S.L.U.Prescription required
Online doctors for ZTALMY 50 mg/mL ORAL SUSPENSION
Discuss questions about ZTALMY 50 mg/mL ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions