
ZOLADEX TRIMESTRAL 10.8 mg PRE-FILLED SYRINGE IMPLANT

How to use ZOLADEX TRIMESTRAL 10.8 mg PRE-FILLED SYRINGE IMPLANT
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Zoladex Trimestral 10.8 mg implant in preloaded syringe
goserelin
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet:
- What Zoladex Trimestral 10.8 mg is and what it is used for.
- What you need to know before starting to use Zoladex Trimestral 10.8 mg.
- How to use Zoladex Trimestral 10.8 mg.
- Possible side effects.
- Storage of Zoladex Trimestral 10.8 mg.
- Package contents and additional information.
1. What Zoladex Trimestral 10.8 is and what it is used for
Zoladex Trimestral 10.8 mg belongs to a group of medications called anti-hormonals, which means it affects the levels of different hormones (natural chemicals produced by the body). In men, it will reduce the levels of the male hormone, testosterone.
Zoladex Trimestral 10.8 mg is used in men to treat certain types of prostate cancer. Zoladex Trimestral 10.8 mg is not indicated for use in women.
2. What you need to know before starting to use Zoladex Trimestral 10.8 mg
Do not use Zoladex Trimestral 10.8 mg
- If you are allergic to goserelin or any of the other components of this medication (listed in section 6).
Warnings and precautions
- Consult your doctor or pharmacist before starting to use Zoladex Trimestral 10.8 mg.
- Before starting treatment with this medication, inform your doctor if:
- you have had difficulty urinating or have suffered from discomfort in the lumbar region of the back,
- you have diabetes or,
- you have high blood pressure (hypertension).
- Medications of this type may cause a loss of calcium from the bones (decrease in their thickness). If you have any disease that affects the strength of your bones or risk factors for osteoporosis [e.g., chronic alcohol abuse, being a smoker, long-term treatment with anticonvulsants (medications for epilepsy or convulsions) or corticosteroids (a type of anti-inflammatory medication), family history of osteoporosis], inform your doctor or nurse.
- Depression has been reported in patients taking Zoladex Trimestral 10.8 mg, which can be severe. If you are taking Zoladex Trimestral 10.8 mg and experience depression, inform your doctor.
- Inform your doctor if you have any heart or blood vessel condition or are being treated for it, including medications to control heart rhythm (arrhythmias). The risk of heart rhythm problems may increase when using Zoladex Trimestral 10.8 mg.
- Inform your doctor immediately if you experience pain and bruising in the abdomen or other symptoms of severe bleeding, such as difficulty breathing, dizziness, low blood pressure, and/or alteration of consciousness, which could be the result of vascular injuries at the injection site produced during the administration of Zoladex Trimestral 10.8 mg.
- Treatment with Zoladex Trimestral 10.8 mg may cause positive results in doping tests.
In case of hospitalization, inform the healthcare staff if you are being treated with Zoladex Trimestral 10.8 mg.
Children and adolescents
Zoladex Trimestral 10.8 mg is not indicated for use in children.
Use of Zoladex Trimestral 10.8 mg with other medications
Inform your doctor, pharmacist, or nurse if you are using, have recently used, or may need to use any other medication.
Zoladex Trimestral 10.8 mg may interfere with some medications used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medications (e.g., methadone (used for pain relief and detoxification from other medications), moxifloxacin (an antibiotic), antipsychotics (used to treat severe mental illnesses)).
Pregnancy and lactation
Zoladex Trimestral 10.8 mg is not indicated for use in women.
Driving and using machines
There is no evidence that Zoladex Trimestral 10.8 mg alters the ability to drive or use machines.
3. How to use Zoladex Trimestral 10.8 mg
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor, pharmacist, or nurse again.
Remember to have your medication administered to you.
Your doctor will indicate the duration of your treatment with Zoladex Trimestral 10.8 mg. Do not stop treatment before your doctor tells you to.
Zoladex Trimestral 10.8 mg will be administered to you as an injection by your doctor or nurse, who will follow the instructions on the package label for correct use.
Zoladex Trimestral 10.8 mg is normally administered by injection under the skin every 3 months.
It is essential that you continue treatment with Zoladex Trimestral 10.8 mg even if you feel well, unless your doctor decides to interrupt it.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
When administering Zoladex Trimestral 10.8 mg, the injection may cause an injury at the administration site, including injury to the blood vessels in the abdomen.In very rare cases, this has caused severe bleeding. Seek immediate medical attention if you notice any of the following symptoms: abdominal pain, abdominal swelling, difficulty breathing, dizziness, low blood pressure, and/or any alteration of consciousness. These could be symptoms of severe bleeding due to accidental injury to an abdominal blood vessel during the administration of Zoladex Trimestral 10.8 mg.
Very common side effects (may affect more than 1 in 10 people)
- hot flashes, sweating. These side effects may continue after stopping treatment with Zoladex Trimestral 10.8 mg.
- decreased sexual desire and impotence.
Common side effects (may affect up to 1 in 10 people)
- increased blood sugar levels.
- tingling or numbness in the fingers of the hands or feet.
- skin rash, which is usually mild and resolves without interrupting treatment.
- decreased heart function, heart attack. The risk of developing them is higher when Zoladex is used with other medications (anti-androgens) to treat prostate cancer.
- changes in blood pressure (elevation or decrease).
- bone pain, usually at the start of treatment with Zoladex Trimestral 10.8 mg. If this happens, inform your doctor, as you may need to be prescribed a medication to relieve the pain.
- weight gain.
- breast swelling.
- reactions at the injection site, such as pain, bruising, bleeding, redness, or swelling in the area, or other reactions.
- loss of bone mineral density (decrease in bone thickness).
- mood changes and depression (in prolonged treatments).
- spinal cord compression.
Uncommon side effects (may affect up to 1 in 100 people)
- hypersensitivity reactions to the medication.
- joint pain.
- breast tenderness.
- mood changes and depression (in short treatments).
- ureteral obstruction (the tube that carries urine from the kidneys to the bladder), which can cause difficulty urinating or discomfort in the lumbar region of the back.
Rare side effects (may affect up to 1 in 1,000 people)
- anaphylactic reaction (severe allergic reaction).
Very rare side effects (may affect up to 1 in 10,000 people)
- psychotic disorders that can cause hallucinations, thought disorders, and personality changes.
- development of a tumor in the pituitary gland (a endocrine gland in the head). If you have a tumor in the pituitary gland, Zoladex Trimestral 10.8 mg may cause bleeding from the tumor. Pituitary tumors can cause headaches, nausea, vision loss, and even loss of consciousness.
Side effects of unknown frequency (cannot be estimated from available data)
- hair loss, especially loss of body hair.
- changes in the electrocardiogram (prolongation of the QT interval).
- changes in blood cell count (observed in a blood test).
- blood clots in the lungs (which cause chest pain and difficulty breathing) and inflammation of the tissue surrounding the lung structures (alveoli) where oxygen is absorbed (interstitial pneumonia) (which causes symptoms such as cough and difficulty breathing).
- liver damage.
Reporting side effects.
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Zoladex Trimestral 10.8 mg
Keep this medication out of sight and reach of children.
Do not store above 25°C.
Keep in the original packaging.
Do not use Zoladex Trimestral 10.8 mg after the expiration date shown on the packaging and the outer packaging after the EXP date. The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Zoladex Trimestral 10.8 mg
- The active ingredient is goserelin (as acetate). Each implant contains 10.8 mg of goserelin.
- The other components are a mixture of high and low molecular weight lactic and glycolic acid copolymers.
Appearance of the product and package contents
The medication is presented in the form of a 10.8 mg implant in a preloaded syringe inside a sealed envelope.
The implant is sterile, cream-colored, and releases the medication in a prolonged manner.
The envelope contains a desiccant inside.
The preloaded syringe has a safety device (blue clip) and a needle protection system.
Marketing authorization holder and manufacturer
Marketing authorization holder
AstraZeneca Farmacéutica Spain, S.A.
C/ Puerto de Somport 21-23
28050 Madrid
Spain
Manufacturer
AstraZeneca AB
Gärtunavägen
SE-152 57 Södertälje
Sweden
Other presentations
Zoladex 3.6 mg: Package containing a 3.6 mg implant in a preloaded syringe inside a sealed envelope, which also contains a desiccant inside. The preloaded syringe has a safety device (red clip) and a needle protection system.
Date of the last revision of this leaflet: February 2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medications and Health Products (AEMPS) http://www.aemps.gob.es/
<----------------------------------------------------------------------------------------------------------------------------------?
This information is intended only for healthcare professionals:
Zoladex Trimestral 10.8 mg should be administered via subcutaneous injection - read and understand all instructions completely before administration.
- Place the patient in a comfortable position, with the upper body slightly elevated. Clean the abdominal injection site with a cotton swab impregnated with a disinfectant agent (alcohol, etc...).
NOTE: Caution should be exercised when administering Zoladex Trimestral 10.8 mg in the anterior abdominal wall, due to the proximity of the underlying inferior epigastric artery and its branches. Very thin patients may have a high risk of vascular injury.
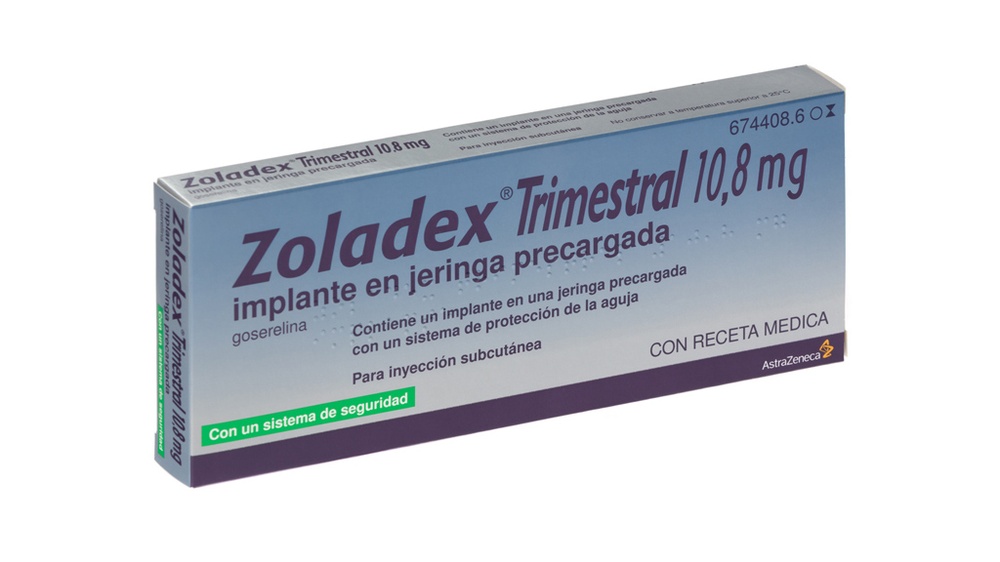
- Examine the envelope and syringe for damage. Remove the syringe from the open envelope and hold it at an angle towards the light.
Check that part of the Zoladex Trimestral 10.8 mg implant is visible. (Figure 1).
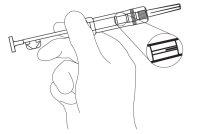 Figure 1
Figure 1
- Gently remove the blue plastic safety tab from the syringe and discard it. (Figure 2).
Remove the cap that protects the needle. As it is not a liquid injectable, there is no need to eliminate air bubbles, as attempting to do so may cause the Zoladex Trimestral 10.8 mg implant to shift.
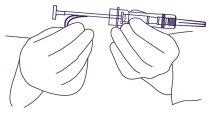 Figure 2
Figure 2
- Hold the syringe around the protection system, using an aseptic technique. Take a skin fold of the patient and insert the needle at a slight angle (30 to 45 degrees).
With the needle opening facing up, insert the needle into the subcutaneous tissueof the anterior abdominal wall below the navel, until the protection system touches the patient's skin. (Figure 3).
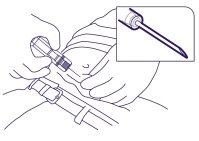 Figure 3
Figure 3
NOTE: The Zoladex Trimestral 10.8 mg syringe cannot be used for aspiration. If the hypodermic needle penetrates a large vessel, blood will be seen immediately in the syringe chamber. If a vessel is penetrated, remove the needle and control any resulting bleeding, monitoring the patient for any signs or symptoms of abdominal bleeding. After ensuring the patient is hemodynamically stable, another Zoladex Trimestral 10.8 mg implant can be injected with a new syringe in another area. Exercise caution when administering Zoladex Trimestral 10.8 mg in patients with a low BMI and/or those receiving full anticoagulant doses.
- Do not penetrate the muscle or peritoneum. In Figure 4, below, an incorrect grip and exposure angle are shown.
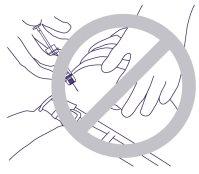 Figure 4
Figure 4
- Press the plunger completely, until it cannot be pressed further, in order to deposit the Zoladex Trimestral 10.8 mg implant and activate the protection system. You may hear a "click" and feel the protection system activate, sliding automatically to cover the needle. If the plunger is not pressed completely, the protection system WILL NOT activate.
NOTE:The needle does not retract.
- Continue holding the syringe as shown in Figure 5, remove the needle, allowing the protection system to continue sliding and covering the needle.
Discard the syringe in a container, in accordance with local regulations.
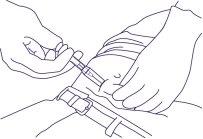 Figure 5
Figure 5
NOTE: In the unlikely event of the need for surgical removal of a Zoladex Trimestral 10.8 mg implant, it can be located by ultrasound.
- Country of registration
- Average pharmacy price380.93 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZOLADEX TRIMESTRAL 10.8 mg PRE-FILLED SYRINGE IMPLANTDosage form: IMPLANT, 3.6 mgActive substance: goserelinManufacturer: Astrazeneca Farmaceutica Spain S.A.Prescription requiredDosage form: INJECTABLE, 42 mgActive substance: leuprorelinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription required
Online doctors for ZOLADEX TRIMESTRAL 10.8 mg PRE-FILLED SYRINGE IMPLANT
Discuss questions about ZOLADEX TRIMESTRAL 10.8 mg PRE-FILLED SYRINGE IMPLANT, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












