
ZAVESCA 100 mg HARD CAPSULES

How to use ZAVESCA 100 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Zavesca 100 mg Capsules
miglustat
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Zavesca and what is it used for
- What you need to know before you take Zavesca
- How to take Zavesca
- Possible side effects
- Storage of Zavesca
- Contents of the pack and other information
1. What is Zavesca and what is it used for
Zavesca contains the active substance miglustat which belongs to a group of medicines that have an effect on metabolism. It is used to treat two disorders:
- Zavesca is used to treat mild to moderate type 1 Gaucher disease in adults.
In type 1 Gaucher disease, your body cannot break down a substance called glucosylceramide. As a result, it accumulates in some cells of your immune system. This can lead to enlargement of the liver and spleen, changes in the blood, and effects on the bones.
The usual treatment for type 1 Gaucher disease is enzyme replacement therapy (ERT). Zavesca will only be used in cases where enzyme replacement therapy is not suitable for the patient.
- Zavesca is also used to treat the progressive neurological symptoms of Niemann-Pick disease type C in adults and children.
If you have Niemann-Pick disease type C, lipids such as glycosphingolipids can accumulate in the cells of your brain. This can lead to impairment of neurological functions such as slow eye movements, balance, swallowing, memory, or seizures.
Zavesca works by inhibiting the enzyme called glucosylceramide synthase, which is responsible for the first step in the synthesis of most glycosphingolipids.
2. What you need to know before you take Zavesca
Do not take Zavesca
- if you are allergic to miglustat or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before starting Zavesca
- if you have kidney disease
- if you have liver disease
Your doctor will perform the following tests before and during your treatment with Zavesca:
- physical examination of the nerves in your arms and legs
- measurement of vitamin B12 levels
- monitoring of growth in children or adolescents with Niemann-Pick disease type C
- monitoring of platelet count in blood
These tests are necessary because some patients have reported symptoms such as tingling or numbness in hands and feet, or a reduction in body weight during treatment with Zavesca. The tests will help your doctor determine if these effects are due to your disease or to pre-existing conditions or are side effects of Zavesca (see section 4 for more details).
If you have diarrhea, your doctor may ask you to modify your diet to reduce lactose and carbohydrate intake such as sucrose (cane sugar), or not to take Zavesca with food, or to temporarily reduce the dose. In some cases, your doctor may prescribe medications to treat diarrhea, such as loperamide. Talk to your doctor if diarrhea does not respond to these measures or if you experience any other abdominal discomfort. In this case, your doctor may decide to perform additional tests.
Male patients are advised to use reliable contraceptive methods during treatment with Zavesca and for three months after stopping treatment.
Children and adolescents
Do not give this medicine to children and adolescents (under 18 years of age) with type 1 Gaucher disease because it is not known if it works in this disease.
Other medicines and Zavesca
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Tell your doctor if you are taking medicines that contain imiglucerase, which are sometimes used at the same time as Zavesca. They may decrease the amount of Zavesca in your body.
Pregnancy, breastfeeding, and fertility
Do not take Zavesca if you are pregnant or planning to become pregnant. For more information, talk to your doctor or pharmacist. You must use an effective contraceptive method while taking Zavesca. You should not breastfeed while taking Zavesca.
Male patients should use a reliable contraceptive method during treatment with Zavesca and for three months after stopping treatment.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, talk to your doctor or pharmacist before using this medicine.
Driving and using machines
Zavesca may cause dizziness. Do not drive or use tools or machines if you feel dizzy.
Zavesca contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per capsule; this is essentially "sodium-free".
3. How to take Zavesca
Follow exactly the instructions of administration of this medicine given to you by your doctor. If you are not sure, talk to your doctor or pharmacist again.
- For type 1 Gaucher disease: In adults, the usual dose is one capsule (100 mg) three times a day (morning, afternoon, evening), which is a maximum daily dose of three capsules (300 mg).
- For Niemann-Pick disease type C: In adults and adolescents (over 12 years), the usual dose is 2 capsules (200 mg) three times a day (morning, afternoon, and evening). This is a maximum daily dose of six capsules (600 mg).
For children under 12 years, your doctor will adjust the dose for Niemann-Pick disease type C.
In case of kidney problems, your doctor may prescribe a lower initial dose. It is possible that your doctor may reduce the dose of Zavesca to one capsule (100 mg) one or two times a day if you experience diarrhea during treatment with Zavesca (see section 4). Your doctor will tell you how long to take Zavesca.
To remove the capsule:
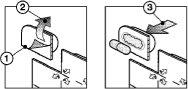
- Separate at the perforated line
- Remove the paper as indicated by the arrows
- Remove the capsule by pressing through the aluminum
Zavesca can be taken with or without food. You should swallow the capsule whole with a glass of water.
If you take more Zavesca than you should
If you take more capsules than you were told to, talk to your doctor immediately. In clinical trials, Zavesca has been used at doses of up to 3000 mg: this caused a reduction in blood leukocytes and other side effects similar to those described in section 4.
If you forget to take Zavesca
Take the next capsule when it is due. Do not take a double dose to make up for forgotten doses.
If you stop taking Zavesca
Do not stop taking Zavesca without talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
More serious side effects:
Some patients have reported tingling or numbness in hands and feet (this occurs frequently).These could be signs of peripheral neuropathy, due to side effects of Zavesca or could be due to pre-existing conditions. Your doctor will perform tests before and during treatment with Zavesca to assess your case (see section 2).
If you experience any of these effects, tell your doctor as soon as possible.
If you experiencea mild tremor, usually tremor in the hands, tellyour doctor as soon as possible. The tremor usually resolves without the need to stop treatment. In some cases, your doctor may need to reduce the dose and even stop treatment with Zavesca to control the tremor.
Very common:(may affect more than 1 in 10 people)
The most common side effects are diarrhea, flatulence (gas), abdominal pain (stomach), weight loss, and decreased appetite.
If you lose some weightwhen starting treatment with Zavesca, do not worry, people usually stop losing weight with continued treatment.
Common:(may affect up to 1 in 10 people)
Common side effects are headache, dizziness, paresthesia (tingling or numbness), coordination problems, hypoesthesia (reduced sensation to touch), dyspepsia (indigestion), nausea (feeling sick), constipation, and vomiting, abdominal swelling or discomfort (stomach) and thrombocytopenia (reduced blood platelet levels). Neurological symptoms and thrombocytopenia may be due to the underlying disease.
Other possible side effects are muscle spasms or weakness, fatigue, chills, and feeling unwell, depression, difficulty sleeping, memory loss, and decreased libido.
Most patients notice one or more of these side effects, usually when starting treatment or at different times during treatment. Most are mild and resolve quickly. If any of these side effects bother you, talk to your doctor. He or she may reduce the dose of Zavesca or prescribe other medicines to control the side effects.
Reporting of side effects
Ifyou experience side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zavesca
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after "EXP". The expiry date refers to the last day of the month shown.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Zavesca
The active substanceis miglustat 100 mg.
The other ingredients are:
Sodium starch glycolate,
Povidone (K30),
Magnesium stearate.
Gelatin,
Titanium dioxide (E171).
Black iron oxide (E172),
Lacquer.
Appearance and packaging
Zavesca is a white 100 mg capsule with "OGT 918" printed in black on the cap and "100" printed in black on the body.
Box with four blister strips, each strip containing 21 capsules, for a total of 84 capsules.
Marketing authorisation holder:
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer:
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
You can request more information about this medicine from the local representative of the marketing authorisation holder.
België/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
| Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Tel: +49 2137 955 955 | Nederland Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Ελλáδα Janssen-Cilag Φαρμακευτικn Α.Ε.Β.Ε. Tηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen-Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
| Sverige Janssen-Cilag AB Tfn: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom (Northern Ireland) Janssen Sciences Ireland UC Tel: +44 1 494 567 444 |
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites about rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZAVESCA 100 mg HARD CAPSULESDosage form: CAPSULE, 100 mgActive substance: miglustatManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: CAPSULE, 100 mgActive substance: miglustatManufacturer: Dipharma Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 100 mgActive substance: miglustatManufacturer: Gen.Orph S.A.S.Prescription required
Online doctors for ZAVESCA 100 mg HARD CAPSULES
Discuss questions about ZAVESCA 100 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions

















