
WELIREG 40 mg FILM-COATED TABLETS

How to use WELIREG 40 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
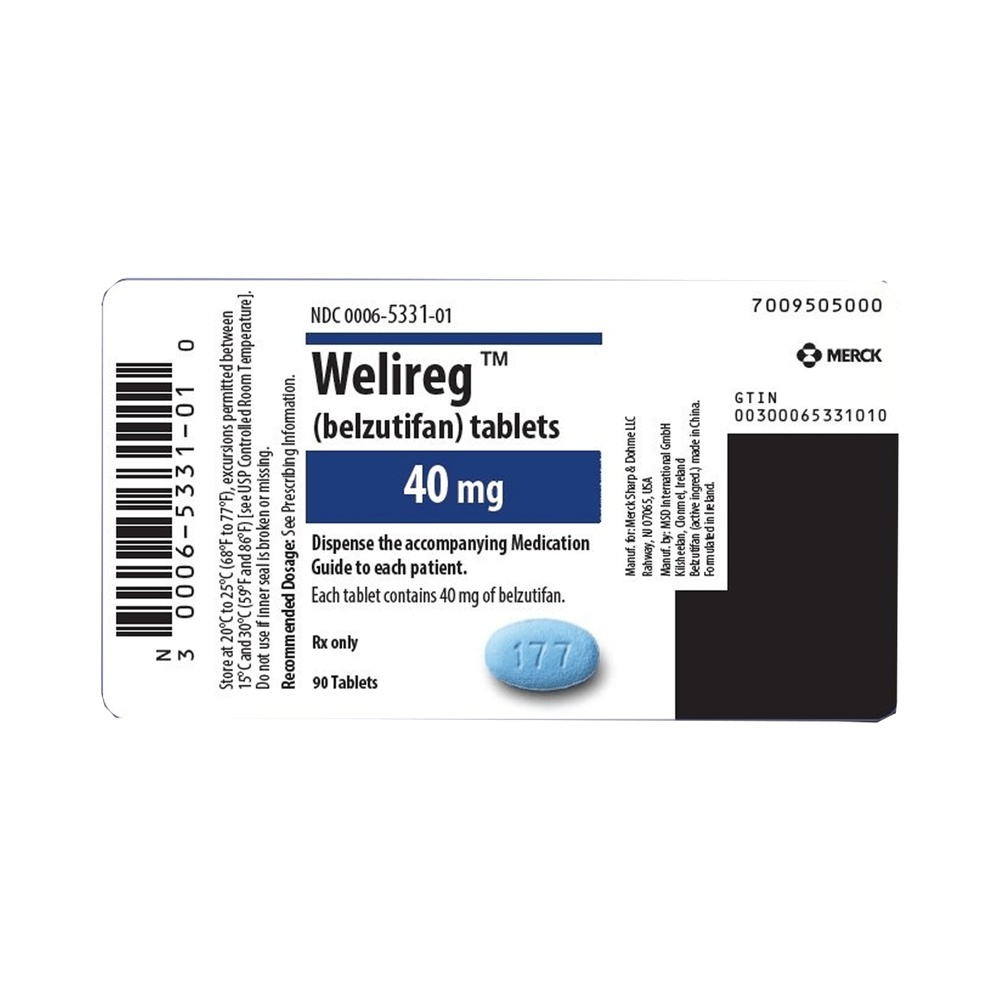
WELIREG 40mg film-coated tablets
belzutifan
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- The pack also contains a patient information card for women who could become pregnant. Please read it as it contains important safety information that you need to know before and during treatment with WELIREG.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is WELIREG and what is it used for
- What you need to know before you take WELIREG
- How to take WELIREG
- Possible side effects
- Storage of WELIREG
- Contents of the pack and other information
1. What is WELIREG and what is it used for
WELIREG is a cancer medicine that contains the active substance belzutifan.
WELIREG is used to treat adults with:
- Renal cell carcinoma with a clear cell component, a type of kidney cancer. It is used when the cancer is advanced (has spread) after treatments that act on the immune system (PD-1 or PD-L1 inhibitors) and the blood vessels of the cancer (VEGF-directed therapy).
- Von Hippel-Lindau disease (a genetic disorder that produces tumors and cysts that grow in certain parts of the body) that requires treatment for renal cell carcinoma, for brain and spinal cord tumors called central nervous system hemangioblastomas, or for a type of pancreatic cancer called pancreatic neuroendocrine tumor, and for which surgery or other local procedures are not suitable.
The active substance in WELIREG, belzutifan, blocks a protein called hypoxia-inducible factor 2 alpha (HIF-2α). This protein helps control how cells and blood vessels grow, which plays an important role in the development and spread of tumors in the body.
2. What you need to know before you take WELIREG
Do not take WELIREG
- If you are allergic to belzutifan or any of the other ingredients of this medicine (listed in section 6). Talk to your doctor or pharmacist if you are not sure.
- If you are pregnant and need to be treated for Von Hippel-Lindau disease (see section "Pregnancy").
Warnings and precautions
Talk to your doctor or pharmacist before you start taking WELIREG:
- If you have respiratory problems
- If you have low red blood cell counts (anemia)
- If you have Von Hippel-Lindau disease and tumors in the brain and spinal cord
Low red blood cell counts (anemia)
Treatment with WELIREG may cause anemia (low red blood cell counts). Tell your doctor if you develop any of the following symptoms:
- difficulty breathing
- feeling tired (fatigue)
- dizziness
- pale skin
Your doctor will check you for anemia before you start treatment with WELIREG and during treatment. If you develop severe anemia, your doctor may start treatment with medicines that stimulate the production of red blood cells (erythropoiesis-stimulating agents) and/or blood transfusions and stop treatment with WELIREG until the anemia is resolved, or may permanently stop treatment with WELIREG.
Lower oxygen levels in your blood (hypoxia)
Treatment with WELIREG may cause hypoxia. Tell your doctor immediately if you develop any of the following symptoms:
- difficulty breathing
- rapid heartbeats
- rapid breathing
- bluish discoloration of the skin around the mouth
- inability to say complete sentences without taking a breath
- unusual tiredness
- confusion
Your doctor will check you for hypoxia before you start treatment with WELIREG and during treatment. If you develop severe hypoxia, your doctor may start treatment with oxygen therapy or stop treatment with WELIREG. Treatment with WELIREG will be restarted at a lower dose. If it happens again, your doctor will stop treatment with WELIREG.
In some cases, if you develop very severe hypoxia, your doctor may permanently stop treatment with WELIREG.
Bleeding in your brain and spinal cord (central nervous system hemorrhage)
Treatment with WELIREG for Von Hippel-Lindau disease may cause bleeding in your brain and spinal cord if you have tumors in the brain and/or spinal cord. Tell your doctor immediately if you develop any of the following symptoms:
- severe headache
- vision problems
- severe drowsiness
- severe weakness on one side of your body
- uncoordinated muscle movements
- severe pain in the neck or back
- loss of sensation of pain, temperature, and touch
Children and adolescents
WELIREG is not recommended for use in children and adolescents under 18 years of age. It is not known if WELIREG is safe and effective in these patients.
Other medicines and WELIREG
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This is because WELIREG may affect how other medicines work. Also, some other medicines may affect how WELIREG works.
WELIREG may affect how hormonal contraceptives work. While you are taking WELIREG and for at least one week after the last dose, you should:
- use a non-hormonal effective contraceptive method or
- have your male partner use a condom.
Pregnancy
Do not take WELIREG for Von Hippel-Lindau disease if you are pregnant.
If you are pregnant and need treatment for renal cell carcinoma, talk to your doctor about using WELIREG.
WELIREG may harm your baby and may cause a miscarriage. If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Your doctor will do a pregnancy test before you start taking WELIREG.
You should not become pregnant while taking WELIREG.
If you are a woman who can become pregnant:
- Hormonal contraceptives, such as birth control pills, injections, or patches, may not work as well while you are taking WELIREG.
- While you are taking WELIREG and for at least one week after the last dose, you should:
- use a non-hormonal effective contraceptive method or
- have your male partner use a condom.
Talk to your doctor or pharmacist about the contraceptive methods that may be suitable for you while taking WELIREG.
Fertility
WELIREG may cause fertility problems in men and women, which may affect your ability to have children. Talk to your doctor or pharmacist if this worries you.
Breast-feeding
Tell your doctor or pharmacist if you are breast-feeding or plan to breast-feed.
It is not known if WELIREG passes into breast milk. Taking this medicine while breast-feeding may harm your baby.
You and your doctor will decide together if you should take WELIREG or breast-feed, but not both at the same time.
If you want to start breast-feeding, wait at least 1 week after the last dose of WELIREG.
Driving and using machines
You may feel dizzy or tired while taking WELIREG. If this happens, do not drive or use machines until you feel better.
WELIREG contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per film-coated tablet, which is essentially "sodium-free".
3. How to take WELIREG
Take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, talk to your doctor or pharmacist.
How much to take
- The recommended dose is 120 mg once a day. Take three 40 mg tablets once a day, at about the same time every day.
- Your doctor may reduce the dose or stop treatment, either for a short time or permanently, if you experience certain side effects while taking WELIREG (see section 4).
How to take it
Swallow the tablets whole, do not break them. It is not known if this medicine works if the tablets are not taken whole.
You can take WELIREG with or without food.
If you take more WELIREG than you should
If you take too many tablets, talk to a doctor or go to a hospital.
If you forget to take WELIREG
If you miss a dose of WELIREG, take the missed dose as soon as possible on the same day. Take your usual dose of WELIREG the next day.
If you vomit after taking WELIREG, do not take another dose. Take your usual dose of WELIREG the next day.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
WELIREG may cause the following side effects, which may be serious (see section 2):
- low red blood cell counts (anemia) (very common, may affect more than 1 in 10 people)
- lower oxygen levels in your blood (hypoxia) (very common, may affect more than 1 in 10 people)
- difficulty breathing (dyspnea) (very common, may affect more than 1 in 10 people)
Tell your doctor if you notice any of the following symptoms:
- feeling tired (fatigue)
- pale skin
- shortness of breath
- difficulty breathing
- chest pain
- rapid heartbeats
- or dizziness
You may need a blood transfusion if your red blood cell count is too low. You may need oxygen therapy if your oxygen levels in the blood are too low.
Your doctor will do blood tests to check your red blood cell count and measure the oxygen level in your blood before and during treatment with WELIREG.
Other side effects that may occur:
Very common (may affect more than 1 in 10 people):
- feeling tired (fatigue)
- feeling dizzy
- feeling sick (nausea)
- bleeding (hemorrhage) (including bleeding in your brain and spinal cord if you have central nervous system hemangioblastomas associated with Von Hippel-Lindau disease)
Common (may affect up to 1 in 10 people):
- weight gain
Tell your doctor or pharmacist if you notice any of the side effects mentioned above.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of WELIREG
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister and carton after "EXP". The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions.
Do not use this medicine if the packaging is damaged or shows signs of tampering.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Packaging Contents and Additional Information
Composition of WELIREG
- The active substance is belzutifan. Each film-coated tablet contains 40 mg of belzutifan.
- The other ingredients are sodium croscarmellose (E 468) (see "WELIREG contains sodium" in section 2), hypromellose acetate succinate, magnesium stearate (E 470b), mannitol (E 421), microcrystalline cellulose (E 460), and anhydrous colloidal silica (E 551). The film coating contains aluminum carmine indigo lake (E 132), macrogol (E 1521), polyvinyl alcohol (E 1203), talc (E 553b), and titanium dioxide (E 171).
Appearance and Packaging of the Product
WELIREG is a film-coated, oval tablet, blue in color, with "177" engraved on one side and smooth on the other. WELIREG is available in aluminum/aluminum blisters. Each pack contains 30 film-coated tablets. Each multiple pack contains 90 film-coated tablets (3 packs of 30). Not all pack sizes may be available in your country.
Marketing Authorization Holder and Manufacturer
Merck Sharp & Dohme B.V.
Waarderweg 39
2031 BN Haarlem
Netherlands
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien MSD Belgium Tel: +32(0)27766211 | Lithuania UAB Merck Sharp & Dohme Tel: + 370 5 278 02 47 |
| Luxembourg/Luxemburg MSD Belgium Tél/Tel: +32(0)27766211 |
Czech Republic Merck Sharp & Dohme s.r.o. Tel: +420 233 010 111 | Hungary MSD Pharma Hungary Kft. Tel.: +36 1 888 5300 |
Denmark MSD Danmark ApS Tlf.: + 45 4482 4000 | Malta Merck Sharp & Dohme Cyprus Limited Tel: 8007 4433 (+356 99917558) |
Germany MSD Sharp & Dohme GmbH Tel: +49 (0) 89 20 300 4500 | Netherlands Merck Sharp & Dohme B.V. Tel: 0800 9999000 (+31 23 5153153) |
Estonia Merck Sharp & Dohme OÜ Tel: +372 6144 200 | Norway MSD (Norge) AS Tlf: +47 32 20 73 00 |
Greece MSD Α.Φ.Ε.Ε Τηλ: +30 210 98 97 300 | Austria Merck Sharp & Dohme Ges.m.b.H. Tel: +43 (0) 1 26 044 |
Spain Merck Sharp & Dohme de España, S.A. Tel: +34 91 321 06 00 | Poland MSD Polska Sp. z o.o. Tel: +48 22 549 51 00 |
France MSD France Tél: + 33 (0) 1 80 46 40 40 | Portugal Merck Sharp & Dohme, Lda Tel: +351 21 4465700 |
Croatia Merck Sharp & Dohme d.o.o. Tel: + 385 1 6611 333 | Romania Merck Sharp & Dohme Romania S.R.L. Tel: +40 21 529 29 00 |
Ireland Merck Sharp & Dohme Ireland (Human Health) Limited Tel: +353 (0)1 2998700 | Slovenia Merck Sharp & Dohme, inovativna zdravila d.o.o. Tel: +386 1 5204 201 |
Iceland Vistor hf. Sími: + 354 535 7000 | Slovak Republic Merck Sharp & Dohme, s. r. o. Tel: +421 2 58282010 |
Italy MSD Italia S.r.l. Tel: 800 23 99 89 (+39 06 361911) | Finland MSD Finland Oy Puh/Tel: +358 (0)9 804 650 |
Cyprus Merck Sharp & Dohme Cyprus Limited Τηλ.: 800 00 673 (+357 22866700) | Sweden Merck Sharp & Dohme (Sweden) AB Tel: +46 77 5700488 |
Latvia SIA Merck Sharp & Dohme Latvija Tel.: + 371 67025300 |
Date of last revision of this leaflet:
This medicinal product has been authorized with a "conditional approval". This type of approval means that more information on this medicinal product is expected.
The European Medicines Agency will review the new information on this medicinal product at least once a year and this leaflet will be updated as necessary.
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to WELIREG 40 mg FILM-COATED TABLETSDosage form: CAPSULE, 0.5 mgActive substance: anagrelideManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: CAPSULE, 0.5 mgActive substance: anagrelideManufacturer: Bluefish Pharmaceuticals Ab (Publ)Prescription requiredDosage form: CAPSULE, 0.5 mgActive substance: anagrelideManufacturer: Glenmark Arzneimittel GmbhPrescription required
Online doctors for WELIREG 40 mg FILM-COATED TABLETS
Discuss questions about WELIREG 40 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







