How to use VOYDEYA 100 MG FILM-COATED TABLETS
Introduction
Patient Information Leaflet
Voydeya 50mg film-coated tablets
Voydeya 100mg film-coated tablets
danicopan
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Voydeya and what is it used for
- What you need to know before you take Voydeya
- How to take Voydeya
- Possible side effects
- Storage of Voydeya
- Contents of the pack and other information
1. What is Voydeya and what is it used for
What is Voydeya
Voydeya contains the active substance danicopan. Danicopan blocks a protein called factor D, which is part of the body's defense system known as the "complement system". By blocking factor D, danicopan prevents the complement system from instructing the immune system to destroy red blood cells (hemolysis).
What Voydeya is used for
Voydeya is used to treat adult patients with paroxysmal nocturnal hemoglobinuria (PNH) who are receiving treatment with another type of medicine for PNH known as a C5 inhibitor (ravulizumab or eculizumab) and have residual hemolytic anemia (low red blood cell count due to destruction by the immune system). Voydeya is given in addition to ravulizumab or eculizumab.
2. What you need to know before you take Voydeya
Do not take Voydeya
- If you are allergic to danicopan or any of the other ingredients of this medicine (listed in section 6).
- If you have not been vaccinated against meningococcal infection.
- If you have a meningococcal infection.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with this medicine.
Severe infections
Before starting treatment with Voydeya, tell your doctor if you have any infection.
Meningococcal infections
Since the medicine acts on the complement system, which is part of the body's defense system against infections, the use of this medicine may increase the risk of a meningococcal infection caused by Neisseria meningitidis. This is a serious infection that affects the membranes of the brain and can cause brain inflammation (encephalitis) and spread through the blood and body (sepsis).
Consult your doctor before starting treatment with this medicine to ensure you are up to date with Neisseria meningitidisvaccines at least 2 weeks before starting treatment. If it is not possible to vaccinate 2 weeks before, your doctor will prescribe antibiotics to reduce the risk of infection until 2 weeks after vaccination. If you have received these vaccines in the past, you may need additional vaccines (booster shots) before starting treatment with Voydeya. You should also be aware that vaccination does not always prevent this type of infection.
The following are symptoms of a meningococcal infection. If you experience any of these symptoms, inform your doctor immediately:
- Headache with nausea (feeling sick) or vomiting
- Headache and fever
- Headache with stiff neck or back
- Fever
- Fever and rash
- Confusion
- Muscle pain with flu-like symptoms
- Sensitivity to light
Treatment of meningococcal infection during travel
If you travel to an area where you cannot contact your doctor or receive temporary medical treatment, your doctor may prescribe an antibiotic against Neisseria meningitidisfor you to take with you. If you experience any of the above symptoms, you should take the antibiotic treatment as directed. You should still see a doctor as soon as possible, even if you feel better after taking the antibiotics.
Other severe infections
According to national recommendations, your doctor may consider that you need additional measures to prevent any other infection.
Kidney problems
Consult your doctor if you have severe kidney problems. Your doctor may need to adjust your dose and monitor you during treatment with Voydeya due to high levels of danicopan in the blood.
Low body weight
Consult your doctor if you have a low body weight below 60 kg. Your doctor may need to monitor you during treatment with Voydeya due to high levels of danicopan in the blood.
Blood tests
The medicine may increase the amount of certain liver enzymes in the blood. Your doctor will perform some blood tests to check your liver before starting treatment. Voydeya is not recommended in patients with severe liver failure.
Children and adolescents
Do not give this medicine to children under 18 years of age, as there is no data on its safety and efficacy in this age group.
Other medicines and Voydeya
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, you should tell your doctor if you are taking any of the following medicines, so that your doctor can decide if it is necessary to change your treatment:
- Dabigatran and edoxaban, medicines to prevent blood clots
- Digoxin, a medicine to treat irregular heartbeats
- Fexofenadine, a medicine to treat allergy symptoms
- Tacrolimus, a medicine used to suppress the immune system
- Rosuvastatin, a medicine used to lower cholesterol levels in the blood
- Sulfasalazine, a medicine used to treat inflammatory bowel disease or rheumatoid arthritis
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
The effects of this medicine on the fetus are unknown. As a precaution, do not take Voydeya if you are pregnant.
This medicine may be excreted in breast milk. Do not use Voydeya during breastfeeding. You should not start breastfeeding until 3 days after stopping treatment with Voydeya.
Driving and using machines
Voydeya has no or negligible influence on the ability to drive and use machines.
Voydeya contains lactose monohydrate
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
Voydeya contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per tablet, which is essentially "sodium-free".
3. How to take Voydeya
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
How much to take
The recommended initial dose of Voydeya is 150 mg three times a day, with an approximate interval of 8 hours between doses (2 hours before or after). Your doctor may decide to increase the dose to 200 mg three times a day based on how you respond to treatment.
If you have severe kidney disease, the recommended initial dose of Voydeya is 100 mg three times a day, with an approximate interval of 8 hours between doses (2 hours before or after). Your doctor may decide to increase the dose to 150 mg three times a day based on how you respond to treatment.
Based on the prescribed dose, the number of tablets per dose will be as follows:
- 100 mg: one 100 mg tablet
- 150 mg: one 50 mg tablet and one 100 mg tablet
- 200 mg: two 100 mg tablets
How to take this medicine
You should take the tablets with food (meal or snack).
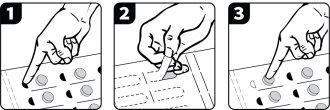
If you were given Voydeya in a blister pack, follow these instructions to remove the tablets from the packaging:
- Press the black semicircle.
- Turn the blister pack over and tear off the tab to expose the aluminum.
- Press on the plastic blister to remove the tablet.
If you take more Voydeya than you should
If you have taken more Voydeya than you should, talk to your doctor immediately. Bring the medicine pack with you so that you can easily describe what you have taken.
If you forget to take Voydeya
If you miss a dose, take it as soon as possible. If it is almost time for your next dose, skip the missed dose and take the next dose at the usual time. Do not take a double dose to make up for forgotten doses.
If you stop taking Voydeya
Do not stop taking Voydeya unless your doctor tells you to. If you stop taking this medicine, the symptoms of residual hemolytic anemia may return. If you need to stop taking this medicine, your doctor will gradually reduce your dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
If you experience any of the symptoms of meningococcal infection (see section 2, "Symptoms of meningococcal infection"), inform your doctor immediately:
- Headache with nausea (feeling sick) or vomiting
- Headache and fever
- Headache with stiff neck or back
- Fever
- Fever and rash
- Confusion
- Muscle pain with flu-like symptoms
- Sensitivity to light
Other side effects
Verycommon(may affect more than 1 in 10 people)
- Fever or elevated temperature (pyrexia)
- Headache
- Blood test showing increased levels of liver enzymes
Common(may affect up to 1 in 10 people)
- Pain in the arms and legs (pain in the extremities)
- Vomiting
- High blood pressure
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in the Yellow Card Scheme: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Voydeya
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister pack after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions. After first opening of the bottle, use the medicine within 48 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Voydeya contains
The active substance is danicopan. Each film-coated tablet contains 50 or 100 mg of danicopan.
The other ingredients are:
- Tablet core: lactose monohydrate, microcrystalline cellulose, sodium croscarmellose, sodium lauryl sulfate, magnesium stearate, hydrophobic colloidal silica, hypromellose acetate succinate. See section 2 Voydeya contains lactose monohydrate and sodium.
- Coating: polyvinyl alcohol, titanium dioxide (E171), macrogol 4000, talc.
Appearance and packaging
Voydeya 50 mg film-coated tablets are white to off-white, round, film-coated tablets with "DCN" above "50" engraved on one side and no engraving on the other.
Voydeya 100 mg film-coated tablets are white to off-white, round, film-coated tablets with "DCN" above "100" engraved on one side and no engraving on the other.
The tablets are packaged in a bottle or blister pack.
Bottle
- Voydeya 50 mg film-coated tablets + Voydeya 100 mg film-coated tablets: each pack contains 180 tablets (1 bottle with 90 tablets of 50 mg and 1 bottle with 90 tablets of 100 mg).
- Voydeya 100 mg film-coated tablets: each pack contains 180 tablets (2 bottles with 90 tablets of 100 mg each).
Blister pack
- Voydeya 50 mg film-coated tablets + Voydeya 100 mg film-coated tablets: each pack contains 168 tablets (4 blister packs, each with 21 tablets of 50 mg and 21 tablets of 100 mg).
- Voydeya 100 mg film-coated tablets: each pack contains 168 tablets (4 blister packs, each with 42 tablets of 100 mg).
Not all pack sizes may be marketed.
Marketing authorisation holder
Alexion Europe SAS
103-105, rue Anatole France
92300 Levallois-Perret
France
Manufacturer
Alexion Pharma International Operations Limited
College Business and Technology Park
Blanchardstown Road North
Dublin 15
D15 R925
Ireland
You can request more information about this medicine from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Alexion Pharma Belgium Tél/Tel: +32 0 800 200 31 | Lietuva UAB AstraZeneca Lietuva Tel: +370 5 2660550 |
| Luxembourg/Luxemburg Alexion Pharma Belgium Tél/Tel: +32 0 800 200 31 |
Ceská republika AstraZeneca Czech Republic s.r.o. Tel: +420 222 807 111 | Magyarország AstraZeneca Kft. Tel.: +36 1 883 6500 |
Danmark Alexion Pharma Nordics AB Tlf.: +46 0 8 557 727 50 | Malta Alexion Europe SAS Tel: +353 1 800 882 840 |
Deutschland Alexion Pharma Germany GmbH Tel: +49 (0) 89 45 70 91 300 | Nederland Alexion Pharma Netherlands B.V. Tel: +32 (0)2 548 36 67 |
Eesti AstraZeneca Tel: +372 6549 600 | Norge Alexion Pharma Nordics AB Tlf: +46 (0)8 557 727 50 |
Ελλάδα AstraZeneca A.E. Τηλ: +30 210 6871500 | Österreich Alexion Pharma Austria GmbH Tel: +41 44 457 40 00 |
España Alexion Pharma Spain, S.L. Tel: +34 93 272 30 05 | Polska AstraZeneca Pharma Poland Sp. z o.o. Tel.: +48 22 245 73 00 |
France Alexion Pharma France SAS Tél: +33 1 47 32 36 21 | Portugal Alexion Pharma Spain, S.L. - Sucursal em Portugal Tel: +34 93 272 30 05 |
Hrvatska AstraZeneca d.o.o. Tel: +385 1 4628 000 | România AstraZeneca Pharma SRL Tel: +40 21 317 60 41 |
Ireland Alexion Europe SAS Tel: +353 1 800 882 840 | Slovenija AstraZeneca UK Limited Tel: +386 1 51 35 600 |
Ísland Alexion Pharma Nordics AB Sími: +46 0 8 557 727 50 | Slovenská republika AstraZeneca AB, o.z. Tel: +421 2 5737 7777 |
Italia Alexion Pharma Italy srl Tel: +39 02 7767 9211 | Suomi/Finland Alexion Pharma Nordics AB Puh/Tel: +46 0 8 557 727 50 |
Κύπρος Alexion Europe SAS Τηλ: +357 22490305 | Sverige Alexion Pharma Nordics AB Tel: +46 0 8 557 727 50 |
Latvija SIA AstraZeneca Latvija Tel: +371 67377100 |
Date of last revision of this leaflet:01/2025.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site: https://www.ema.europa.eu. There are also links to other web sites about rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VOYDEYA 100 MG FILM-COATED TABLETSDosage form: TABLET, 50 mg + 100 mgActive substance: danicopanManufacturer: Alexion Europe SasPrescription requiredDosage form: INJECTABLE PERFUSION, 1080 mgActive substance: pegcetacoplanManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE INFUSION, 300 mgActive substance: eculizumabManufacturer: Amgen Technology (Ireland) Unlimited CompanyPrescription required
Online doctors for VOYDEYA 100 MG FILM-COATED TABLETS
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for VOYDEYA 100 MG FILM-COATED TABLETS – subject to medical assessment and local rules.