
VOQUILY 1mg/mL ORAL SOLUTION

How to use VOQUILY 1mg/mL ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Voquily 1 mg/ml Oral Solution
melatonin
Read all of this leaflet carefully before you or your child start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you or your child only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Voquily and what is it used for
- What you need to know before you take Voquily
- How to take Voquily
- Possible side effects
- Storage of Voquily
- Contents of the pack and further information
1. What is Voquily and what is it used for
Voquily contains the active substance melatonin, which is a hormone produced naturally by the body. This hormone helps regulate the body's day and night rhythm.
Voquily may be used for sleep onset insomnia in children and adolescents (6-17 years) with Attention Deficit Hyperactivity Disorder (ADHD) where other sleep habits have not worked well enough.
2. What you need to know before you take Voquily
Do not take Voquily
- if you are allergic to melatonin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medicine
- if you have epilepsy. This medicine may increase the frequency of seizures in patients with epilepsy
- if you have an autoimmune disease (where the body is 'attacked' by its own immune system)
- if you have diabetes or glucose intolerance, as this medicine may increase blood glucose levels (see section 3)
- if you have impaired liver or kidney function
- if you smoke. Smoking may reduce the effect of this medicine, as tobacco smoke components may increase the breakdown of melatonin by the liver
- if you are an elderly person
- if you are a woman of childbearing potential. Contraceptives should be used during treatment with this medicine. However, this medicine may be affected by certain contraceptives; see the section 'Other medicines and Voquily' for more information.
Children under 6 years
Do not give this medicine to children under 6 years as its safety and efficacy are unknown.
Other medicines and Voquily
Tell your doctor or pharmacist if you or your child are taking, have recently taken or might take any other medicines. These medicines are:
- Fluvoxamine (used to treat depression and obsessive-compulsive disorder), as it may increase the effect of melatonin
- Psoralens (used to treat skin disorders such as psoriasis), as they may increase the effect of melatonin
- Cimetidine (used to treat stomach problems such as ulcers), as it may increase the effect of melatonin
- Estrogens (used in contraceptives or hormone replacement therapy), as they may increase the effect of melatonin
- Quinolones (used to treat bacterial infections), as they may increase the effect of melatonin
- Rifampicin (used to treat bacterial infections), as it may decrease the effect of melatonin
- Smoking may decrease the effect of melatonin
- Carbamazepine (used to treat epilepsy), as it may decrease the effect of melatonin
- Betablockers (used to treat hypertension), as they may reduce the effects of melatonin
- Nifedipine (used to treat hypertension), as melatonin may reduce the effect of nifedipine
- Benzodiazepines and non-benzodiazepine hypnotics (medicines used to induce sleep, e.g. midazolam, temazepam, zaleplon, zolpidem, zopiclone), as melatonin may enhance the sedative effect of these drugs, as well as enhance certain side effects of zolpidem (morning somnolence, nausea, confusion)
- Warfarin (anticoagulants), as melatonin may affect the effect of the anticoagulant warfarin
- Thioridazine (used to treat mental disorders), as taken together with melatonin increases the feeling of somnolence and difficulty performing tasks
- Imipramine (used to treat depression), as taken together with melatonin increases the feeling of somnolence and difficulty performing tasks
- Caffeine (stimulant), as melatonin interacts with caffeine.
Voquily with food, drinks, and alcohol
- Do not drink alcohol before, during, or after taking this medicine, as it increases somnolence when taken with melatonin.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, consult your doctor or pharmacist before using this medicine.
Contraceptives for women of childbearing potential and young girls
Women of childbearing potential and young girls should use contraceptives when taking Voquily. Since some contraceptives may increase melatonin levels in the body, the choice of contraceptive should be discussed with a doctor (see 'Other medicines and Voquily').
Pregnancy
Voquily is not recommended if you or your daughter is pregnant. Melatonin crosses the placenta and there is not enough information on the risk this may pose to the fetus.
Breastfeeding
Voquily is not recommended if you or your daughter is breastfeeding. Melatonin passes into human breast milk and a risk to the breastfed child cannot be excluded.
Driving and using machines
This medicine may cause somnolence and may reduce alertness for several hours after intake. Therefore, this medicine should not be taken before driving or using machines.
Voquily contains sorbitol and propylene glycol
This medicine contains 140 mg of sorbitol in each ml. Sorbitol is a source of fructose. If your doctor has told you (or your child) that you have an intolerance to some sugars or have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disorder in which the patient cannot break down fructose, consult your doctor before taking or receiving this medicine.
This medicine contains 150 mg of propylene glycol in each ml.
3. How to take Voquily
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended initial dose is 1-2 ml (1-2 mg) 30 to 60 minutes before bedtime.
The dose will be adjusted individually up to a maximum of 5 ml (5 mg) per day, regardless of age. The lowest possible dose will be administered.
Treatment should be monitored by a doctor (recommended at least every 6 months) to see if it is still appropriate. Treatment should be interrupted once a year to see if it is still necessary.
Diabetes
If you or your child has diabetes or glucose intolerance, do not consume food 2 hours before or 2 hours after taking Voquily, see 'Warnings and precautions'.
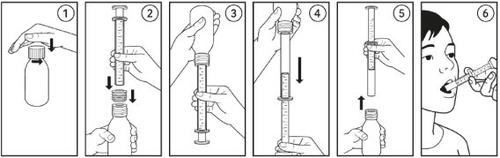
Instructions for use
Voquily should be swallowed with a glass of water.
Do not consume food 1 hour before or 1 hour after taking the medicine.
A 10 ml oral syringe with intermediate graduations of 0.5 ml and a 'press-in' adapter for the bottle are provided with the medicine.
- Open the bottle and insert the adapter into the bottle opening (1-2) when using it for the first time.
- Insert the syringe into the adapter (2-3) and turn the bottle upside down.
- Draw out the required volume from the inverted bottle (4).
- Put the bottle back in a vertical position and remove the filled syringe from the adapter (5).
- Slowly insert the contents of the syringe into the mouth by pushing the syringe and swallow the medicine (6).
- Clean the syringe and put the cap back on to close the bottle (the adapter remains in place).
If you take more Voquily than you should
If you or your child has accidentally taken too much medicine or, for example, your child has taken the medicine by mistake, contact a doctor or pharmacist as soon as possible, or call the Toxicology Information Service, telephone 915620420, stating the medicine and the amount taken.
The most common symptoms of overdose are somnolence, headache, dizziness, and nausea.
If you forget to take Voquily
If you forget to take your dose at bedtime and wake up during the night, you can take the missed dose but no later than 04:00 in the morning.
Do not take a double dose to make up for forgotten doses.
If you stop taking Voquily
No harmful effects are known if treatment is stopped or finished. It is not known whether the use of Voquily causes withdrawal effects after treatment is finished.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you or your child experience any of the following serious side effects, tell your doctor immediatelyand stop taking this medicine.
Frequency not known
- Hypersensitivity reaction (allergic reactions, such as itching, difficulty breathing)
- Inflammation of deeper skin layers (angioedema)
- Inflammation of the mouth and tongue (edema)
The following are other side effects that may occur.
Common side effects(may affect up to 1 in 10 people)
- Headache
- Somnolence
Uncommon side effects(may affect up to 1 in 100 people)
- Irritability, nervousness, restlessness, insomnia
- Abnormal dreams, nightmares, night sweats, anxiety, anxious restlessness, weakness, lack of energy and enthusiasm
- Migraine
- Dizziness
- Hypertension
- Abdominal pain, mouth ulceration, dry mouth, nausea
- Skin disorders (dermatitis, itching, rash, dry skin)
- Pain in arms and legs
- Menopausal symptoms
- Chest pain
- Glucose in the urine, excess protein in the urine
- Changes in blood composition that may cause yellowing of the skin and eyes
- Abnormal liver function tests
Rare side effects(may affect up to 1 in 1,000 people)
- Herpes zoster
- Decreased number of white blood cells in the blood
- Decreased number of platelets in the blood
- Low calcium or sodium levels in the blood
- High levels of fats in the blood
- Changes in mood, aggression, agitation, crying, stress symptoms, feeling of confusion (disorientation), early morning awakenings, increased sexual desire (increased libido), depressed mood, depression
- Fainting, memory impairment, attention disturbance, dreamlike state, feeling of discomfort in the legs (restless legs syndrome), poor quality sleep, fatigue
- Visual impairment, blurred vision, increased tearing
- Feeling of dizziness or spinning (vertigo), dizziness when standing or sitting
- Fast heartbeat, chest pain due to angina
- Acid reflux, gastrointestinal disorder, mouth ulcers, tongue ulcers, stomach upset, vomiting, abnormal bowel sounds, increased salivation, bad breath, flatulence, abdominal discomfort, stomach inflammation
- Abnormal skin sensation (paresthesia), skin disorders (eczema, erythema, psoriasis), nail disorder, sudden feeling of heat (hot flush)
- Pain, arthritis, muscle spasms, neck pain, nocturnal cramps
- Frequent urination, presence of red blood cells in the urine, need to urinate at night
- Prolonged erection (priapism), prostate inflammation (prostatitis)
- Thirst
- Increased liver enzymes, abnormal blood electrolytes, abnormal laboratory tests
Not known(frequency cannot be estimated from the available data)
- Spontaneous flow of milk from the breasts (also in males)
Other side effects in children and adolescents
A low frequency of side effects has been reported, which are generally mild. The most common side effects were headache, hyperactivity, feeling of dizziness or spinning (vertigo), and abdominal pain. No serious side effects have been observed.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines and Healthcare Products Agency (AEMPS) website (http://www.aemps.gob.es). By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Voquily
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging and on the label after EXP. The expiry date is the last day of the month stated.
Store in the original package to protect from light.
This medicine does not require any special storage temperature.
After first opening: use within 6 months.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to a pharmacy for disposal. Ask your pharmacist how to dispose of containers and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Voquily
- 1 ml contains 1 mg of melatonin.
- The other ingredients are:
- propylene glycol (E1520), non-crystallizing liquid sorbitol (E420), sucralose (E955), strawberry flavor (including propylene glycol [E1520]), purified water, hydrochloric acid (for pH adjustment) (E507).
Appearance of Voquily and contents of the pack
Voquily is a clear, colorless to yellowish solution with a strawberry odor. The medicine is packaged in a brown glass bottle with a child-resistant screw cap. A 10 ml oral syringe with intermediate graduations of 0.5 ml and a 'press-in' adapter for the bottle are also provided.
Pack size: 60 ml or 150 ml.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Clinigen Healthcare B.V.
Schiphol Boulevard 359
WTC Schiphol Airport,
D Tower 11th floor
1118BJ Schiphol
Netherlands
Manufacturer
Rafarm S.A.
Thesi Pousi-Xatzi
Agiou Louka
Paiania Attiki
19002, PO Box 37
Greece
This medicine is authorized in the Member States of the European Economic Area under the following names:
Sweden | Voquily |
Austria | VOQUILY 1 mg/ml Lösung zum Einnehmen |
Belgium | Voquily 1 mg/ml drank solution buvable Lösung zum Einnehmen |
France | VOQUILY 1 mg/ml solution buvable |
Germany | VOQUILY 1 mg/ml Lösung zum Einnehmen |
Hungary | VOQUILY 1 mg/ml belsoleges oldat |
Ireland | Voquily 1 mg/ml oral solution |
Italy | VOQUILY 1 mg/ml soluzione orale |
Netherlands | VOQUILY 1 mg/ml drank |
Poland | VOQUILY |
Spain | Voquily 1 mg/ml solución oral |
Date of last revision of this leaflet:February 2024
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) (http://www.aemps.gob.es).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VOQUILY 1mg/mL ORAL SOLUTIONDosage form: MODIFIED-RELEASE TABLET, 2 mgActive substance: melatoninManufacturer: Rad Neurim Pharmaceuticals Eec S.A.R.L.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 2 mgActive substance: melatoninManufacturer: Neurim Pharmaceuticals Ltd.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 2 mgActive substance: melatoninManufacturer: Arrotex Pharmaceuticals LimitedPrescription required
Online doctors for VOQUILY 1mg/mL ORAL SOLUTION
Discuss questions about VOQUILY 1mg/mL ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















