
VIMKUNYA INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE

How to use VIMKUNYA INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
VIMKUNYA injectable suspension in a pre-filled syringe
chikungunya vaccine (recombinant, adsorbed)
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start receiving this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is VIMKUNYA and what is it used for
- What you need to know before you receive VIMKUNYA
- How VIMKUNYA is administered
- Possible side effects
- Storage of VIMKUNYA
- Contents of the pack and other information
1. What is VIMKUNYA and what is it used for
VIMKUNYA is a vaccine used to prevent disease caused by the chikungunya virus in people aged 12 years and older.
VIMKUNYA is a vaccine that contains part of the "outer shell" of the chikungunya virus. This "outer shell" is not infectious and cannot cause chikungunya, but it teaches the immune system (the body's natural defenses) to protect itself against the virus that causes chikungunya.
Chikungunya is a disease caused by the chikungunya virus, which is spread through the bite of infected mosquitoes. This disease is present in countries in Asia, Africa, and the subtropical regions of America. Most people infected with the virus develop fever, rash, and severe joint pain, which usually disappear within one to two weeks, but can last for months or years.
2. What you need to know before you receive VIMKUNYA
You must not receive VIMKUNYAif you are allergic to the active substance or to any of the other components of this vaccine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before receiving VIMKUNYA if:
- you have ever had a severe allergic reaction (hypersensitivity) or breathing problems after receiving any other vaccine.
- you have ever fainted after receiving an injection.
- you have a severe illness or infection with high fever (above 38°C). You can receive the vaccine if you have a mild fever or a mild upper respiratory tract infection such as a cold.
- you have a weakened immune system (immunodeficiency) or are taking medicines that weaken the immune system (such as high-dose corticosteroids, immunosuppressants or anticancer medicines).
- you have a bleeding or bruising problem (such as thrombocytopenia or haemophilia) or are taking an anticoagulant (a medicine to prevent blood clots).
Children
VIMKUNYA must not be used in children under 12 years of age. There is no information on the use of VIMKUNYA in this age group.
Other medicines and VIMKUNYA
Tell your doctor if you are taking, have recently taken or might take any other medicines or vaccines.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before receiving this vaccine.
Driving and using machines
Some of the possible side effects of VIMKUNYA mentioned in section 4 of this leaflet may temporarily affect your ability to drive or use machines. Wait until the effects of the vaccine have worn off before driving or using machines.
VIMKUNYA contains sodium and potassium
This vaccine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
This vaccine contains less than 1 mmol of potassium (39 mg) per dose; this is essentially "potassium-free".
3. How VIMKUNYA is administered
VIMKUNYA is given as a single injection into the large muscle of the upper arm. It is preferable to receive the injection in the non-dominant arm.
If you have any other questions about the use of this vaccine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Seek medical attention immediately if you have symptoms of a severe allergic reaction after receiving the vaccine. The symptoms can be:
- feeling faint or dizzy
- changes in heart rate
- difficulty breathing
- wheezing ("whistling" while breathing)
- swelling of the lips, face or throat
- swelling with itching under the skin (urticaria) or skin rash
- nausea or vomiting
- stomach pain
After receiving this vaccine, the following side effects can also occur:
Very common:may affect more than 1 in 10 people
- pain at the injection site
- fatigue (tiredness)
- headache
- muscle pain
Common:may affect up to 1 in 10 people
- chills
- joint pain (arthralgia)
- general feeling of being unwell
- nausea
Uncommon:may affect up to 1 in 100 people
- redness, bruising or swelling at the injection site
- fever
- dizziness
- nasal congestion
- skin rash
Rare(may affect up to 1 in 1,000 people):
- tingling (paresthesia)
- limb pain
- diarrhoea
- swelling of the lips
- swollen lymph nodes (adenopathy)
- diarrhoea and vomiting (gastroenteritis)
- sore throat (oropharyngeal pain)
- runny nose (rhinorrhoea)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of VIMKUNYA
Keep this vaccine out of the sight and reach of children.
Your doctor, pharmacist or nurse is responsible for storing this medicine and for disposing of any unused medicine correctly. This information is intended for healthcare professionals.
Do not use this vaccine after the expiry date which is stated on the label after EXP.
Store in a refrigerator (between 2°C and 8°C). Do not freeze. Keep the syringe in the outer packaging to protect it from light.
The in-use stability data indicate that the vaccine is stable for 4 hours when stored at temperatures between 8°C and 25°C and for at least 24 hours when stored at temperatures between 0°C and 2°C. After this period, the vaccine must be discarded.
Medicines should not be disposed of via wastewater or household waste. Your doctor, pharmacist or nurse will dispose of this vaccine. This will help protect the environment.
6. Contents of the pack and other information
What is in VIMKUNYA
Each dose of 0.8 ml contains 40 micrograms of pseudoviral particle proteins of the chikungunya virus (CHIKV) adsorbed on hydrated aluminium hydroxide.
1 Produced in human embryonic kidney cells using recombinant DNA technology.
2 Derived from the Senegal 37997 strain of CHIKV, consisting of the capsid protein (C) and the envelope proteins E1 and E2 of CHIKV.
Aluminium content per 0.8 ml dose: approximately 300 micrograms of Al3+.
Hydrated aluminium hydroxide is included in the vaccine as an adjuvant. Adjuvants are substances included in certain vaccines to accelerate, improve or prolong the protective effects of the vaccine.
The other ingredients (excipients) are: sucrose, potassium dihydrogen phosphate, dipotassium phosphate, sodium citrate and water for injections.
See section 2 "VIMKUNYA contains sodium and potassium".
Appearance and packaging
1 dose of VIMKUNYA injectable suspension contains 0.8 ml.
Package size: 1 pre-filled syringe.
Before shaking, the vaccine is a clear liquid with a white precipitate.
Marketing authorisation holder
Bavarian Nordic A/S
Philip Heymans Alle 3
DK-2900 Hellerup
Denmark
Manufacturer
Bavarian Nordic A/S
Hejreskovvej 10 A
DK-3490 Kvistgaard
Denmark
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website https://www.ema.europa.eu.
You can also scan the QR code with a mobile device to get the leaflet in different languages or visit the URL
QR code to be included
------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Administer VIMKUNYA only by the intramuscular route, preferably in the deltoid muscle of the upper arm. Do not administer by the intravenous, intradermal or subcutaneous route.
Dosage
A single intramuscular dose of 0.8 ml should be administered.
Instructions for handling and administration
Do not use this vaccine after the expiry date which is stated on the label after EXP. The expiry date is the last day of the month shown.
The vaccine must be handled by a healthcare professional using aseptic technique to ensure the sterility of each dose.
Do not mix VIMKUNYA with any other vaccine in the same syringe or vial.
Storage conditions:
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Keep the syringe in the outer packaging to protect it from light.
Preparation for use:
- Remove the vaccine carton from the refrigerator (between 2°C and 8°C).
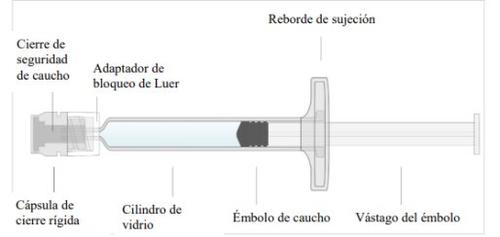
Inspection of the pre-filled syringe
- Remove the syringe tray from the carton.
- Remove the pre-filled syringe from the tray by holding the syringe cylinder.
- Inspect the pre-filled syringe for any abnormal appearance or leakage. If you notice any defect, do not use the pre-filled syringe.
- VIMKUNYA is a clear liquid with a white precipitate before shaking.
- Shake the pre-filled syringe vigorously just before use to obtain a homogeneous suspension. After shaking, the suspension should be a turbid white liquid without any visible foreign particles. Inspect the suspension for any change in colouration and the presence of particles. Do not administer the vaccine if you notice any of these signs.
Administration of the vaccine
- Hold the syringe cylinder with the cone pointing upwards and gently unscrew the Luer lock adapter from the pre-filled syringe. Do not attempt to break or remove the tip, as this may damage the syringe.
- This container does not contain a needle. Use a sterile needle of suitable size to ensure intramuscular injection, depending on the patient's size and weight.
- Attach the sterile needle to the pre-filled syringe and ensure that the needle is securely fitted to the syringe.
- After shaking, VIMKUNYA is a homogeneous turbid white suspension without any visible foreign particles. If the vaccine is not a homogeneous suspension, shake the syringe vigorously to resuspend the solution before administration.
- Administer the full dose as an intramuscular (IM) injection into the deltoid muscle of the upper arm by gently pressing the plunger and maintaining pressure on the plunger until the entire contents of the syringe have been expelled to complete the injection.
- VIMKUNYA is administered only by the intramuscular (IM) route. Do not administer by the intravenous, intradermal or subcutaneous route.
- The injection should be administered within 4 hours of removing the pre-filled syringe from the refrigerator (between 2°C and 8°C).
- The in-use stability data indicate that the vaccine is stable for 4 hours when stored at temperatures between 8°C and 25°C and for at least 24 hours when stored at temperatures between 0°C and 2°C. After this period, the vaccine must be discarded.
Disposal
- Discard the syringe after use.
- Discard this vaccine if it is not used within 4 hours of removing the pre-filled syringe from the storage place at a temperature between 2°C and 8°C.
Elimination
- Disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local requirements.
- Country of registration
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VIMKUNYA INJECTABLE SUSPENSION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 60 micrograms/dose + 60 micrograms/doseActive substance: respiratory syncytial virus vaccinesManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 0.5 mLActive substance: respiratory syncytial virus vaccinesManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 50 µgActive substance: respiratory syncytial virus vaccinesManufacturer: Moderna Biotech Spain S.L.Prescription required
Online doctors for VIMKUNYA INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE
Discuss questions about VIMKUNYA INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















