
VERSATIS 700 MG MEDICATED ADHESIVE DRESSING

How to use VERSATIS 700 MG MEDICATED ADHESIVE DRESSING
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Versatis 700 mg Medicinal Adhesive Patch
Lidocaine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Versatis and what is it used for
- What you need to know before you use Versatis
- How to use Versatis
- Possible side effects
- Storage of Versatis
- Contents of the pack and further information
1. What is Versatis and what is it used for
Versatis contains lidocaine, a local anesthetic, which acts by reducing pain in the skin.
You have been given Versatis to treat a painful skin problem called post-herpetic neuralgia. This problem is usually characterized by localized symptoms such as burning pain, stabbing pain, or shooting pain.
2. What you need to know before you use Versatis
Do not use Versatis
- if you are allergic to lidocaine or any of the other ingredients of this medicine (listed in section 6),
- if you have had an allergic reaction to other products similar to lidocaine, such as bupivacaine, etidocaine, mepivacaine, or prilocaine,
- on damaged skin or open wounds.
Warnings and precautions
Consult your doctor or pharmacist before you start using Versatis.
If you have a severe liver disease, or severe heart problems, or severe kidney problems, you should talk to your doctor before using Versatis.
Versatis should only be used on areas of the skin after the skin has healed completely. It should not be used in or near the eyes or mouth.
Lidocaine is broken down in the liver into several compounds. One of these compounds is 2,6-xylidine, which has been shown to produce tumors in rats when administered for life at very high doses. The importance of these findings in humans is unknown.
Children and adolescents
Versatis has not been studied in patients under 18 years of age. Therefore, it is not recommended for use in this patient population.
Using Versatis with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Versatis should not be used during pregnancy, unless clearly necessary.
No studies have been conducted with the adhesive patch in breastfeeding women. When using Versatis, only very small amounts of the active ingredient lidocaine may be present in the blood. It is unlikely to have an effect on breastfed babies.
Driving and using machines
It is unlikely that Versatis will affect your ability to drive or use machines. Therefore, you can drive or operate machinery while using Versatis.
Versatis contains propylene glycol, methyl parahydroxybenzoate, and propyl parahydroxybenzoate
The patches contain propylene glycol (E1520), a substance that may cause skin irritation. They also contain methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216), substances that may cause allergic reactions. Sometimes, allergic reactions can occur after using the patch for some time.
3. How to use Versatis
Follow exactly the administration instructions of this medicine given by your doctor. If you are in doubt, consult your doctor or pharmacist again.
The recommended daily dose consists of covering the painful areas of the skin using one to three patches. Versatis can be cut into smaller pieces to fit the affected area. Do not use more than 3 patches at the same time.
The patches should be removed after 12 hours of use, and a 12-hour patch-free period should follow.
You can choose to apply Versatis during the day or during the night.
Usually, you will have some pain relief on the first day you use the patch, but it may take up to 2-4 weeks until you see the full effect of Versatis on pain relief. If after that time you still have a lot of pain, talk to your doctor, because the benefits of treatment should be weighed against the possible risks (see section 2 "Warnings and precautions").
Your doctor will check at regular intervals if Versatis is working well.
Before applying Versatis to the affected area
- If the painful area of the skin has hair, you can cut the hair using scissors. Do not shave.
- The skin must be clean and dry.
- Creams and lotions can be used on the affected skin during the period when you are not using the patch.
- If you have recently taken a bath or shower, you should wait until the skin cools down before using the patch.
Applying the patch
Step 1: Open the envelope and remove one or more patches
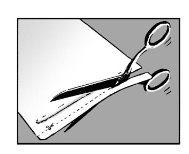
? Open the envelope by tearing or cutting along the dotted line
? When using scissors, be careful not to damage the patches
? Remove one or more patches depending on the size of the painful area of the skin
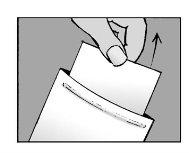
Step 2: Close the envelope
- Close the envelope hermetically after use
- The patch contains water and will dry out if the envelope is not closed properly
Step 3: Cut the patch, if necessary

- If necessary, cut the patch to fit the size of the painful area of the skin before removing the protective liner
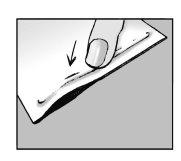
Step 4: Remove the protective liner
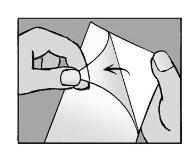
- Remove the transparent liner from the adhesive patch
- Try not to touch the adhesive part of the patch
Step 5: Apply the patch and press firmly on the skin
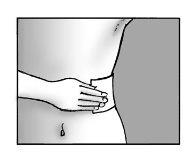
- Apply up to three patches to the painful area of the skin
- Press the patch onto the skin
- Press for at least 10 seconds to ensure the patch adheres firmly
- Make sure the entire patch adheres to the skin, including the edges
Leave the adhesive patch on for only 12 hours
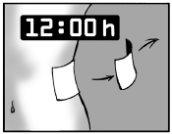
It is important that Versatis is in contact with the skin for only 12 hours. For example, if you have more pain at night, you may want to apply the patch at 7 pm and remove it at 7 am.
If you have more pain during the day than at night, you may want to apply Versatis at 7 am and remove it at 7 pm.
Baths, showers, and swimming
If possible, contact with water should be avoided while using Versatis. You can bathe, shower, or swim during the time when you are not wearing the patch. If you have recently taken a bath or shower, you should wait until the skin cools down before using the patch.
If the patch comes off
Very rarely, the patch may fall off or come loose. If it does, try to reapply it to the same area. If it does not stay in place, remove it and apply a new patch to the same area.
How to remove Versatis
When changing patches, remove the old patch slowly. If it does not come off easily, you can soak it in warm water for a few minutes before removing it.
If you forget to remove the patch after 12 hours
As soon as you remember, remove the old patch. You can use a new patch again after 12 hours.
If you use more patches than you should
If you use more patches than necessary or wear them for too long, this may increase the risk of side effects.
In case of overdose or accidental ingestion, consult the Toxicology Information Service, phone 91 562 04 20.
If you forget to use Versatis
After the 12-hour patch-free period, if you have forgotten to use a new patch, you should apply a new patch as soon as you remember.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Important side effects or symptoms to look out for and what to do if you are affected:
If irritation or a burning sensation occurs while using the adhesive patch, the patch should be removed. The irritated area should remain without a patch until the irritation stops.
Other side effects that may occur:
Very common (may affect more than 1 in 10 people):Skin problems at or around the patch application site, which may include redness, rash, itching, burning, dermatitis, and small blisters.
Uncommon (may affect up to 1 in 100 people):Skin lesions and skin wounds.
Very rare (may affect up to 1 in 10,000 people):Open wound, severe allergic reaction, and allergy.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Versatis
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the envelope and on the carton after "EXP". The expiry date is the last day of the month shown.
Do not refrigerate or freeze.
After first opening: Keep the envelope hermetically closed to protect it from light.
Shelf life after first opening of the envelope: 14 days.
Do not use this medicine if you notice that the envelope has been damaged. If this has happened, the patches may dry out and not adhere properly.
Disposal of Versatis
Used patches still contain active ingredient, which can be harmful to others. Fold used patches in half, joining the adhesive sides, and dispose of them in a way that they are out of the reach of children.
Medicines should not be disposed of via wastewater or household waste. Place the empty packaging and any unused medicine in the SIGRE collection point at your pharmacy. If you have any further questions, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Versatis
- The active substance is lidocaine.
- Each adhesive patch of 14 cm x 10 cm contains 700 mg (equivalent to 5% w/w) of lidocaine.
- The other ingredients of the patch (excipients) are glycerol, liquid sorbitol, sodium carmellose, propylene glycol (E1520), urea, heavy kaolin, tartaric acid, gelatin, polyvinyl alcohol, aluminum glycinates, disodium edetate, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), polyacrylic acid, sodium polyacrylate, purified water.
Supporting tissue and release liner: polyethylene terephthalate (PET)
Appearance of the product and contents of the pack
The medicinal patch measures 14 cm in length and 10 cm in width. It is white and made of non-woven textile material, marked with "Lidocaine 5%". The adhesive patches are packaged in hermetically sealed envelopes, each containing 5 patches.
Each carton contains 5, 10, 20, 25, or 30 patches packaged in 1, 2, 4, 5, or 6 envelopes, respectively. Some pack sizes may not be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Grünenthal Pharma, S.A.
Doctor Zamenhof, 36 – 28027 Madrid
Manufacturer:
Grünenthal GmbH
Zieglerstrasse 6
52078 Aachen
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany, Austria, Belgium, Bulgaria, Cyprus, Denmark, Slovenia, Spain, Estonia, France, Greece, Netherlands, Hungary, Ireland, Iceland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, United Kingdom, Czech Republic, Slovak Republic, Romania, Sweden: Versatis
Portugal: Vessatis
Date of last revision of this leaflet: May 2018.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price77.94 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VERSATIS 700 MG MEDICATED ADHESIVE DRESSINGDosage form: DRESSING, 700 mgActive substance: lidocaineManufacturer: Grünenthal Pharma S.A.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 20 mg/gActive substance: lidocaineManufacturer: Chemische Fabrik Kreussler & Co GmbhPrescription not requiredDosage form: GEL, 20 mg/mlActive substance: lidocaineManufacturer: Farco-Pharma GmbhPrescription required
Online doctors for VERSATIS 700 MG MEDICATED ADHESIVE DRESSING
Discuss questions about VERSATIS 700 MG MEDICATED ADHESIVE DRESSING, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















