
VERASEAL Tissue Adhesive Solutions

How to use VERASEAL Tissue Adhesive Solutions
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
VeraSeal Solutions for Tissue Adhesive
Human fibrinogen/human thrombin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is VeraSeal and what is it used for
- What you need to know before you start using VeraSeal
- How to use VeraSeal
- Possible side effects
- Storage of VeraSeal
- Contents of the pack and further information
1. What is VeraSeal and what is it used for
VeraSeal contains human fibrinogen and human thrombin, two proteins extracted from blood that, when mixed, form a clot.
VeraSeal is used as a tissue adhesive in surgical operations in patients. It is applied to the surface of bleeding tissue to reduce bleeding during and after surgery, when standard surgical techniques are not sufficient.
VeraSeal is indicated for all age groups.
2. What you need to know before you start using VeraSeal
Your surgeon should not administer VeraSeal to you
- if you are allergic to human fibrinogen or human thrombin or to any of the other components of this medicine (listed in section 6).
VeraSeal should not be administered directly into blood vessels.
VeraSeal should not be used to treat severe or rapid bleeding from an artery.
Warnings and precautions
Allergic reactions may occur. The symptoms of these allergic reactions are hives, generalized urticaria, chest tightness, wheezing, hypotension (e.g., dizziness, fainting, blurred vision) and anaphylaxis (a severe, rapidly appearing reaction). If these symptoms occur during surgery, administration of the medicine will be discontinued immediately.
VeraSeal should be applied by spraying only when it is possible to accurately calculate the spraying distance. This spraying distance should be within the recommended range.
Special safety precautions
When medicines are prepared from human blood or plasma, a number of measures are taken to prevent the possible transmission of infections to patients. These measures include careful selection of blood and plasma donors to ensure the exclusion of donors at risk of having infections, and testing of each donation and plasma pool for potential viruses or infections. The manufacturers of these medicines also include a number of stages in the processing of blood or plasma that can inactivate or eliminate viruses. Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of transmitting infections cannot be totally excluded. This also applies to unknown or emerging viruses and other types of infections.
The measures taken are considered effective against enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and against the non-enveloped hepatitis A virus. The measures may be ineffective against non-enveloped viruses such as parvovirus B19. Infection with parvovirus B19 can be severe in pregnant women (fetal infection) and in individuals with immunodeficiency or increased erythropoiesis (e.g., hemolytic anemia).
It is highly recommended that each time VeraSeal is administered to a patient, a record be kept of the name of the medicine and the batch number administered, in order to maintain a link between the patient and the batch of the medicine.
Children and adolescents
The use of VeraSeal is recommended in children and adolescents under 18 years of age.
Using VeraSeal with other medicines
The medicine may be affected if it comes into contact with solutions containing alcohol, iodine, or heavy metals (e.g., antiseptic solutions).
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Your doctor will decide whether you should be treated with VeraSeal.
3. How to use VeraSeal
The use of VeraSeal is restricted to experienced surgeons who have been properly trained in the use of VeraSeal.
Your surgeon will administer VeraSeal to the surface of blood vessels or to the surface of internal organ tissue using the applicator during surgery. This applicator allows the administration of the same amount of the two components of VeraSeal that are to be administered at the same time, and ensures that they are mixed uniformly, which is important for the tissue adhesive to achieve its optimal effect.
The amount of VeraSeal to be applied depends on variables such as the type of surgery, the size of the area to be treated during the operation, and the way VeraSeal is applied. The surgeon will decide the appropriate amount and apply enough to form a thin, uniform layer. If it does not seem sufficient, a second layer can be applied.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
VeraSeal is a fibrin tissue adhesive. Fibrin tissue adhesives in general can cause an allergic reaction in rare cases (up to 1 patient in 1000). If you experience an allergic reaction, you may have one or more of the following symptoms: swelling under the skin (angioedema), skin rash, urticaria or hives, chest tightness, chills, flushing, headache, low blood pressure, lethargy, nausea, restlessness, increased heart rate, tingling, vomiting, or wheezing. In isolated cases, these reactions can progress to a severe allergic reaction. Allergic reactions can be observed especially if the preparation is applied repeatedly or administered to patients known to be allergic to the components of the medicine. If you experience any of these symptoms after surgery, you should consult your doctor or surgeon immediately.
There is also a possibility that your immune system may cause the proteins to attack VeraSeal and interfere with blood coagulation. The frequency of this type of event is unknown.
If this medicine is accidentally placed inside a blood vessel, blood clots may form, including disseminated intravascular coagulation (DIC) (when blood clots form in the blood vessels of the body). There is also a risk of a severe allergic reaction.
The side effects observed during clinical trials with VeraSeal included:
More serious side effects
Uncommon (may affect up to 1 in 100 people):
- Abdominal abscess (inflamed area in the abdomen due to infection)
- Abdominal wound dehiscence (rupture of the wound due to incomplete healing)
- Bile leakage after the procedure
- Cellulitis (skin infection)
- Deep vein thrombosis (blood clots in the blood vessels)
- Hepatic abscess (inflamed area in the liver due to infection)
- Peritonitis (inflammation of the abdominal wall)
- Positive test for parvovirus B19 (laboratory result showing infection with the virus)
- Postoperative wound infection
- Pulmonary embolism (blood clots in the blood vessels of the lungs)
- Wound infection
Other side effects
Common (may affect up to 1 in 10 people):
- Nausea
- Surgical pain
- Pruritus (itching)
Uncommon (may affect up to 1 in 100 people):
- Anemia (lack of red blood cells)
- Anxiety
- Atrial fibrillation (irregular heartbeat)
- Back pain
- Bladder spasms
- Chills
- Conjunctival irritation (eye irritation)
- Constipation
- Contusion (bruising)
- Decreased urine production
- Dyspnea (difficulty breathing)
- Dysuria (pain or difficulty urinating)
- Echymosis (bruising)
- Erythema (redness of the skin)
- Flatulence
- Headache
- High body temperature
- High or low blood pressure
- High or low white blood cell count
- High potassium levels in the blood
- Intestinal obstruction (ileus)
- Abnormal blood coagulation
- Erythema at the incision site (redness of the skin at the incision site)
- Infection at the incision site
- Increased bilirubin levels in the blood
- Increased liver enzyme levels
- Increased or decreased glucose levels in the blood
- Insomnia
- Low blood pressure due to the procedure
- Low calcium levels in the blood
- Low magnesium levels in the blood
- Low oxygen levels in the blood
- Low potassium levels in the blood
- Low protein levels in the blood
- Low red blood cell count due to blood loss
- Low sodium levels in the blood
- Peripheral edema (fluid accumulation)
- Pain, unspecified
- Pain at the incision site
- Pain in the limbs
- Plasma cell myeloma (blood cell cancer)
- Pleurisy (abnormal amount of fluid around the lung)
- Pleural effusion (inflammation of the lung wall)
- Post-procedure hemorrhage (bleeding after the procedure)
- Post-procedure infection (infection after the procedure)
- Pulmonary edema (excess fluid in the lungs)
- Retroperitoneal hematoma (blood accumulation in the abdomen)
- Snores
- Somnolence
- Urinary retention
- Vascular graft complication (vascular bypass complication)
- Vascular graft thrombosis (blood clots in the vascular bypass)
- Ventricular tachycardia (rapid heartbeat)
- Hematoma at the puncture site
- Vomiting
- Wheezing
- Wound secretion
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of VeraSeal
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP.
Store and transport frozen at -18°C or below. The cold chain must not be interrupted until use. Store the sterilized blister in the outer packaging to protect it from light. Thaw completely before use. Once thawed, do not re-freeze. After thawing, it can be stored for no more than 7 days at 2°C - 8°C or 24 hours at a temperature not above 25°C before use.
Once the blister is opened, VeraSeal must be used immediately.
Do not use this medicine if the solution is cloudy or has sediment.
Discard if the packaging is damaged.
6. Package Contents and Additional Information
VeraSeal Composition
The active principles are:
- Component 1: Human Fibrinogen
- Component 2: Human Thrombin
The other components are:
- Component 1: Sodium citrate dihydrate, sodium chloride, arginine, sodium glutamate, water for injectable preparations.
- Component 2: Calcium chloride, human albumin, sodium chloride, glycine, water for injectable preparations.
Product Appearance and Package Contents
VeraSeal is presented as solutions for tissue adhesive in a single-use kit with two pre-filled syringes assembled on a support. Frozen solutions. After thawing, the solutions are transparent or slightly opalescent, colorless or pale yellow.
It includes a Double Applicator with two additional airless spray tips for spray or drop-by-drop application. The airless spray tips are radiopaque. See scheme below.
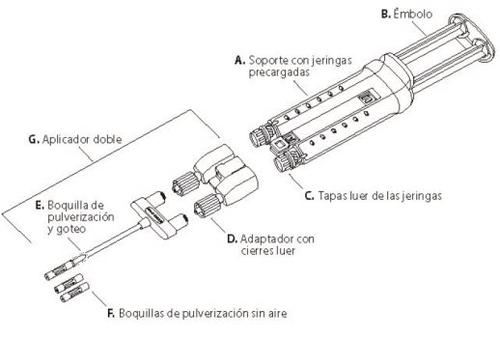
VeraSeal is available in the following presentations:
- VeraSeal 2 ml (contains 1 ml of human fibrinogen and 1 ml of human thrombin)
- VeraSeal 4 ml (contains 2 ml of human fibrinogen and 2 ml of human thrombin)
- VeraSeal 6 ml (contains 3 ml of human fibrinogen and 3 ml of human thrombin)
- VeraSeal 10 ml (contains 5 ml of human fibrinogen and 5 ml of human thrombin)
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Grifols Institute, S.A.
Can Guasc, 2 - Parets del Vallès
E-08150 Barcelona - Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
AT/BE/BG/CY/EE/EL/ES/HR/HU/IE/LV/ LT/LU/MT/NL/RO/SI/SK/UK(NI) Grifols Institute, S.A. Tel: +34 93 571 01 00 | CZ Grifols S.R.O. Tel: +4202 2223 1415 |
DE Grifols Deutschland GmbH Tel: +49 69 660 593 100 | DK/FI/IS/NO/SE Grifols Nordic AB Tel: +46 8 441 89 50 |
FR Johnson & Johnson Medical S.A.S. Tel: +33 (0)1 55 00 22 33 | |
IT Grifols Italia S.p.A. Tel: +39 050 8755 113 | PL Grifols Polska Sp. z o. o. Tel: +48 22 378 85 60 |
PT Grifols Portugal, Lda. Tel: +351 219 255 200 | |
Date of the Last Revision of this Prospectus:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
-------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Dosage and Administration
The use of VeraSeal is limited to experienced surgeons who have been trained in the use of this medication.
The amount of VeraSeal to be applied and the frequency of application should always be guided by the patient's underlying clinical needs.
The dose to be applied will depend on variables such as the type of surgical intervention, the size of the bleeding surface, the severity of the bleeding, the application method chosen by the surgeon, and the number of applications, among others.
The responsible surgeon should apply the medication on an individual basis. In clinical trials, the individual doses administered ranged from 0.3 to 12 ml. For other procedures, larger volumes may be required.
The initial amount of the medication to be applied to a specific anatomical area or surface should be sufficient to completely cover the anticipated application area. If necessary, the application can be repeated.
Incompatibilities
In the absence of compatibility studies, this medication should not be mixed with others.
Special Precautions
Use only on the epithelial layer. Do not administer intravascularly.
Potentially fatal thromboembolic complications may occur if the preparation is inadvertently administered intravascularly.
When using the accessories, follow the instructions for use of these accessories.
Before applying VeraSeal, protect (cover) the parts of the body that are not within the desired application area to avoid tissue adhesion in unwanted areas.
A thin layer of VeraSeal should be applied. If the clot thickness is excessive, it may negatively interfere with the efficacy of the medication and the wound healing process.
Instructions for Use
Read this prospectus before opening the package. Please consult the pictograms at the end of this prospectus.
Instructions for Handling and Disposal of VeraSeal
VeraSeal is presented in sterilized packages and should be used using sterile techniques in aseptic conditions. Discard damaged packages, as they cannot be re-sterilized.
Table 1 shows a summary of the thawing and storage methods after thawing.
Table 1. Thawing and Storage after Thawing
Thawing Method | Thawing Time per Presentation | Storage after Thawing | |
Presentation of 2 ml and 4 ml | Presentation of 6 ml and 10 ml | ||
Refrigerator (2 - 8 °C) | Minimum 7 hours | Minimum 10 hours | 7 days at 2 - 8 °C (refrigerator) in the original package Or 24 hours at a temperature not exceeding 25 °C in the original package |
Thawing at 20 - 25 °C | Minimum 70 minutes | Minimum 90 minutes | |
Sterile water bath (37 °C) in a sterile area | Minimum 5 minutes. Do not exceed 10 minutes. | Minimum 5 minutes. Do not exceed 10 minutes. | Use immediately during surgery |
- Preferred Thawing Methods
Thawing in the refrigerator
- Remove the box from the freezer and place it in the refrigerator for thawing at 2 - 8 °C
for a minimum of 7 hours for 2 ml and 4 ml presentations
for a minimum of 10 hours for 6 ml and 10 ml presentations
After thawing, it is not necessary to heat the medication for use.
After thawing, the solutions are transparent or slightly opalescent, colorless or pale yellow. Solutions that are turbid or have sediment should not be used.
Thawing at 20 °C - 25 °C
Remove the box from the freezer, open it, and remove the two blisters.
Place the blister containing the Double Applicator on a surface at 20 °C - 25 °C until the fibrin tissue adhesive is ready for use.
Thaw the blister with the pre-filled VeraSeal syringes at 20 °C - 25 °C following the steps described below:
- Place the blister containing the syringe support with pre-filled syringes on a surface at 20 °C - 25 °C
for a minimum of 70 minutes for 2 ml and 4 ml presentations
for a minimum of 90 minutes for 6 ml and 10 ml presentations
After thawing, it is not necessary to heat the medication for use.
After thawing, the solutions are transparent or slightly opalescent, colorless or pale yellow. Solutions that are turbid or have sediment should not be used.
Storage after Thawing
After thawing, the kit containing the syringe support with pre-filled VeraSeal syringes and the Double Applicator can be stored for no more than 7 days in the refrigerator at 2 °C - 8 °C or 24 hours at a temperature not exceeding 25 °C before use if it remains sealed in the original package. Once the blisters are opened, use VeraSeal immediately and discard the unused content.
Once thawed, do not re-freeze.
Transfer Instructions
- After thawing, remove the blister from the surface at 20 °C - 25 °C or from the refrigerator at 2 °C - 8 °C.
- Open the blister and check that the pre-filled VeraSeal syringes are completely thawed. Hand the syringe support with pre-filled VeraSeal syringes to a second person to transfer it to the sterile area. The exterior of the blister should not come into contact with the sterile area. See Figure 1.
- Rapid Thawing (Sterile Water Bath)
Remove the box from the freezer, open it, and remove the two blisters.
Place the blister containing the Double Applicator on a surface at 20 °C - 25 °C until the fibrin tissue adhesive is ready for use.
Thaw the pre-filled VeraSeal syringes within the sterile area in a sterile water bath at a temperature of 37 ± 2 °C following the steps described below:
NOTE: Once the VeraSeal blisters are opened, use the product immediately. To avoid the possibility of contamination due to incorrect handling, use a sterile technique and follow the steps indicated below exactly. Do not remove the luer cap from the syringe until thawing is complete and the Double Applicator is ready for assembly.
- Open the blister and hand the syringe support with pre-filled VeraSeal syringes to a second person to transfer it to the sterile area. The exterior of the blister should not come into contact with the sterile area. See Figure 1.
- Place the syringe support with pre-filled syringes directly into the bath, ensuring it remains completely submerged in the sterile water. See Figure 2.
- At 37 °C, the time required is approximately 5 minutes for 2 ml, 4 ml, 6 ml, and 10 ml presentations, but do not leave them at this temperature for more than 10 minutes. The bath temperature should not exceed 39 °C.
- After thawing, dry the syringe support with pre-filled syringes using a sterile surgical gauze.
Confirm that the pre-filled VeraSeal syringes are completely thawed. After thawing, the solutions are transparent or slightly opalescent, colorless or pale yellow. Do not use solutions that are turbid or have sediment.
Use VeraSeal immediately and discard the unused content.
- Assembly Instructions
- Open the blister and hand the Double Applicator VeraSeal and the two additional airless spray tips to a second person to transfer them to the sterile area. The exterior of the blister should not come into contact with the sterile area.
- Hold the syringe support with the luer caps of the syringes pointing upwards. See Figure 3.
- Unscrew and remove the luer caps from the fibrinogen and thrombin syringes. See Figure 3.
- Hold the syringe support with the luer connectors pointing upwards. To eliminate air bubbles from the syringes, gently tap the edge of the syringe support one or two times while keeping it vertical and press the plunger slightly to expel the air. See Figure 4.
- Assemble the Double Applicator. See Figure 5.
NOTE: Do not press the plunger during assembly or before use, as the two biological components would mix prematurely in the airless spray tips, forming a fibrin clot that would prevent product application. See Figure 6.
- Adjust the luer locks and ensure the Double Applicator is correctly assembled. The device is now ready for use.
- Administration
Apply VeraSeal using the syringe support and plunger provided.
Apply VeraSeal using the Double Applicator supplied with the medication. Other application tips (including devices for open and laparoscopic surgery) specifically designed for use with VeraSeal and bearing the CE mark may also be used.
When using the supplied Double Applicator, follow the connection instructions described above. When using other application tips, follow the instructions for use provided with the application tips.
Spray Application
- Hold the Double Applicator and tilt it to the desired position. The tip will retain its shape.
- Place the airless spray tips at a distance of at least 2 cm from the tissue to be treated. Apply firm and regular pressure on the plunger to spray the tissue adhesive. Increase the distance accordingly to achieve the desired coverage of the treatment area.
- If the spray stops for any reason, change the airless spray tip before resuming application, as a clot may have formed in the airless spray tip. To change the airless spray tip, remove the device from the patient and unscrew the used tip. See Figure 7. Place the used airless spray tip away from the replacement airless spray tips. Clean the applicator tip using a dry or moistened sterile surgical gauze. Then, connect a new airless spray tip provided in the packaging and ensure it is firmly connected before use.
NOTE: The red indicator will not be visible if the airless spray tip is correctly connected. See Figure 8.
NOTE: Do not continue pressing the plunger to try to eliminate the fibrin clot from the airless spray tip, as the applicator may become unusable.
NOTE: Do not cut the Double Applicator to avoid exposing the internal wire.
Drop-by-Drop Application
- Remove the airless spray tip from the drop tip by unscrewing it. See Figure 7.
- Hold and tilt the drop tip to the desired position. The tip will retain its shape.
- During drop-by-drop application, keep the tip as close as possible to the tissue surface without touching the tissue during application.
- Apply individual drops to the surface of the area to be treated. To avoid uncontrolled coagulation, allow the drops to separate from each other and from the drop tip.
NOTE: Do not reconnect a used drop tip after it has been removed from the adapter, as a clot may form in the tip and the applicator may become unusable.
- Disposal
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
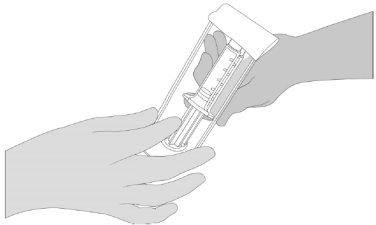
Figure 1
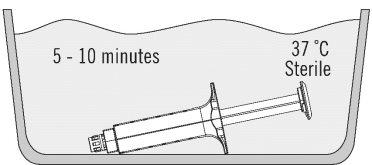
Figure 2
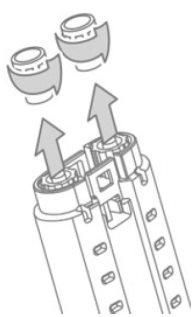
Figure 3
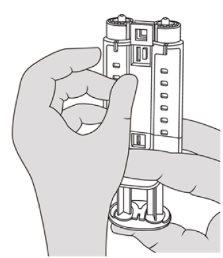
Figure 4
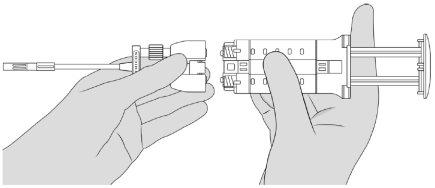
Figure 5
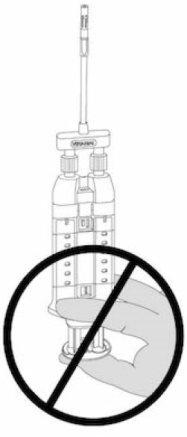
Figure 6
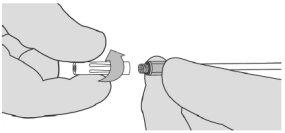
Figure 7
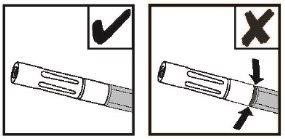
Figure 8
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VERASEAL Tissue Adhesive SolutionsDosage form: TISSUE ADHESIVE, as needed based on the patient's clinical needsActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: TISSUE ADHESIVE, 50-90 mg/ml 800-1200 IUActive substance: combinationsManufacturer: Omrix BiopharmaceuticalsPrescription requiredDosage form: TISSUE ADHESIVE, -Active substance: combinationsManufacturer: Corza Medical GmbhPrescription required
Online doctors for VERASEAL Tissue Adhesive Solutions
Discuss questions about VERASEAL Tissue Adhesive Solutions, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















