
ARTISS Tissue Adhesive Solution, Ultracold

How to use ARTISS Tissue Adhesive Solution, Ultracold
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ARTISS
Tissue Adhesive Solutions
Deep-Frozen
Human Fibrinogen, Human Thrombin, Aprotinin, Calcium Chloride Dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What ARTISS is and what it is used for
- What you need to know before you use ARTISS
- How to use ARTISS
- Possible side effects
- Storage of ARTISS
- Contents of the pack and other information
1. What Artiss is and what it is used for
What ARTISS is
ARTISS is a two-component fibrin sealant that contains two of the proteins that allow blood to clot. These proteins are called fibrinogen and thrombin. When these proteins are mixed during application, they form a clot at the site where the surgeon applies them.
ARTISS is prepared as two solutions (sealant protein solution and thrombin solution), which are mixed when applied.
What ARTISS is used for
ARTISS is a tissue adhesive.
ARTISS is applied to seal soft tissues in plastic, reconstructive, or burn surgery.
For example, ARTISS can be used to attach skin grafts or skin flaps to burn wounds or to attach the skin to the underlying tissue in plastic surgery. ARTISS can also attach artificial skin to wounds.
The clot produced by ARTISS is very similar to the clot that occurs naturally.
This means that it will dissolve naturally without leaving residues. However, aprotinin (a protein that delays the dissolution of clots) is added to increase the duration of the clot and prevent its premature dissolution.
2. What you need to know before you use Artiss
Do not use ARTISS:
- If you are allergic to the active substances or to any of the other components of this medicine (listed in section 6).
- ARTISS should not be used in the case of massive or rapid bleeding.
- ARTISS is not indicated to replace skin sutures made to close a surgical wound.
- ARTISS MUST NOT be injected into blood vessels (veins or arteries) or tissues. As ARTISS forms a clot when applied, injection of ARTISS can cause serious reactions (e.g., vessel occlusion). ARTISS should only be applied to the surface of tissues as a thin layer where necessary.
- You should not receive ARTISS if you are allergic (hypersensitive) to the active substances, bovine proteins, or any of the other components (see section 6) of ARTISS. It can cause serious allergic reactions.
Tell your doctor or surgeon if you know you are allergic to aprotinin or any bovine protein.
- ARTISS should not be applied by spraying in endoscopic procedures. For laparoscopic procedures (minimally invasive surgery), see section "Warnings and precautions".
Warnings and precautions
- Consult your doctor, pharmacist, or nurse before starting to use Artiss.
- There have been cases of gas embolism (air or gas) (introduction of air into the bloodstream that can be fatal or life-threatening) as a result of the use of spray equipment with pressure regulators to apply fibrin tissue adhesives. These cases appear to be related to the use of spray equipment at pressures higher than recommended and/or at a very close distance to the tissue surface. The risk seems to be greater when fibrin tissue adhesives are sprayed with air, compared to CO2, and therefore cannot be excluded with ARTISS.
- When applying ARTISS with a spray device, ensure that both pressure and spray distance are within the recommended range by the manufacturer. ARTISS should be administered exactly as specified in the instructions and only with the equipment recommended for this product.
- Whenever ARTISS is sprayed, changes in blood pressure, pulse, oxygen saturation, andend-tidal CO2 levelshould be monitored to detect possible gas embolism.
- ARTISS should not be used with the Easy Spray/Spray Set system in confined anatomical spaces due to serious safety concerns.
- Artiss is not recommended for laparoscopic surgery (minimally invasive surgery).
- ARTISS should only be applied with CE-marked equipment.
- If accessory nozzles are used with this product, the instructions for use of the nozzles should be followed.
- If you have ever received ARTISS or aprotinin, your body may have developed sensitivity. You may be allergic to this material even if you did not have any reaction during the first application. Inform your doctor if you think you have received any of these products in a previous operation.
- If there are signs of an allergic reaction, your doctor will immediately stop the application of ARTISS and give you the appropriate treatment.
- Artiss is not indicated for stopping bleeding or for sealing in situations where rapid coagulation of the sealant is required. In particular, Artiss should not be used in cardiac surgery procedures where the goal is to seal surgical connections of blood vessels.
- ARTISS is not indicated for use in neurosurgery or as suture support in cases of gastrointestinal or vascular anastomosis, as there are no available data to support these indications.
- Before administration of ARTISS, external body parts outside the application area should be sufficiently protected/covered to prevent any unwanted tissue adhesion.
- ARTISS is applied as a thin layer. An excessively thick clot can negatively affect the efficacy of the product and the wound healing process.
- Your doctor will not use preparations containing oxy cellulose as a transport material, as they may reduce the efficacy of Artiss.
When administering medicines derived from human plasma or blood, certain measures must be taken to prevent infections from being transmitted to patients. Such measures include:
- careful selection of donors to exclude those who are at risk of being carriers of infectious diseases,
- testing for specific infection markers in individual donations and plasma pools,
- as well as inclusion of stages in the manufacturing process to eliminate/inactivate viruses.
Despite this, when administering medicines derived from human blood or plasma, the possibility of transmitting infectious agents cannot be completely excluded. This also applies to emerging or unknown viruses or other types of infections. These measures are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and for non-enveloped viruses such as hepatitis A virus.
The measures taken may have limited value against non-enveloped viruses, such as parvovirus B19. Parvovirus B19 infection can be severe for a pregnant woman (fetal infection) and for individuals whose immune system is depressed or for patients with certain types of anemia (e.g., sickle cell disease or hemolytic anemia).
It is strongly recommended that, each time a dose of ARTISS is administered, a record be kept of the name of the medicine and batch number administered in order to maintain a record of the batches used.
Using ARTISS with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
ARTISS can be used at the same time as other medicines. No interactions between ARTISS and other medicines are known. As with similar products or thrombin solutions, the product may denature if exposed to solutions containing alcohol, iodine, or heavy metals (e.g., antiseptic solutions). Care should be taken to eliminate such substances as much as possible before applying the product.
Using ARTISS with food and drinks
Ask your doctor. The doctor will decide if you can eat or drink before the application of ARTISS.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. Your doctor will decide if ARTISS can be used during pregnancy and breastfeeding.
Driving and using machines
ARTISS will not affect your ability to drive or use machines.
3. How Artiss is used
- ARTISS should only be administered during surgical intervention. The use of ARTISS is
limited to experienced surgeons who have been properly trained in the use of ARTISS.
- The amount of ARTISS to be applied depends on several factors, such as the type of
surgery, the size of the tissue surface to be treated during your operation, and the mode
of application of ARTISS. The surgeon will decide the appropriate amount.
- During your operation, the surgeon will apply ARTISS to the specific tissue using the
special application equipment provided. This equipment ensures that equal amounts of the
two components of the fibrin adhesive are applied at the same time, which is important for
achieving optimal results with ARTISS.
- Before applying ARTISS, it is necessary to dry the wound surface using a standard
technique (e.g., intermittent application of compresses, swabs, use of suction devices).
ARTISS should only be sprayed over visible application areas.
- It is recommended that the initial application cover the entire surface area to be treated.
When applying ARTISS with a spray device, ensure that the pressure and distance to the
tissue are within the recommended ranges by the manufacturer, as indicated below:
Pressure, distance, and recommended equipment for spray application of ARTISS | |||||
Equipment to be used | Applicator tips to be used | Pressure regulator to be used | Distance from target tissue recommended | Recommended spray pressure | |
Open tissue surgery | Tisseel/Artiss spray equipment | n.a. | EasySpray | 10–15 cm | 1.5-2.0 bar (21.5-28.5 psi) |
Tisseel/Artiss spray equipment, 10 | n.a. | EasySpray |
Whenever ARTISS is sprayed, and due to the possibility of gas embolism (air or gas), changes
in blood pressure, pulse, oxygen saturation, and end-tidal CO2 level should be monitored
(see section 2).
If you use more ARTISS than you should
ARTISS will only be applied during a surgical intervention. It is applied by a surgeon, and the amount of ARTISS will be determined by the surgeon.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following table explains the meaning of each frequency, as provided in the following section:
very common: may affect more than 1 in 10 people |
common: may affect up to 1 in 10 people |
uncommon: may affect up to 1 in 100 people |
rare: may affect up to 1 in 1,000 people |
very rare: may affect up to 1 in 10,000 people |
not known: cannot be estimated from the available data |
- There is a slight possibility that you may have an allergic reaction to one of the components of ARTISS (see section 6). This is more likely if you have already been administered ARTISS or aprotinin during a previous operation. Allergic reactions can be serious, so it is very important that you discuss this possibility with your doctor.
- Anaphylactic/anaphylactoid reactions may occur, with a frequency that is not known. The first symptoms of allergic reactions may be: flushing, low blood pressure, increased or decreased heart rate, nausea (feeling unwell), hives, itching, difficulty breathing.
- The surgical team treating you should be aware of the risk of this type of reaction, and if they observe any of these symptoms, they will immediately stop the application of ARTISS. Severe symptoms may require emergency treatment. The frequency of allergic reactions is not known.
- If ARTISS is injected into soft tissues, it may damage local tissues. Frequency not known.
- If ARTISS is injected into blood vessels (veins or arteries), clots (thrombosis) may occur. Frequency not known.
- As ARTISS is manufactured from plasma from blood donations, the risk of infections cannot be completely excluded, although the manufacturer takes numerous measures to reduce the risk (see section 2).
- There have been cases of gas embolism (air or gas) that can be life-threatening or fatal (introduction of air into the bloodstream that can be serious or life-threatening) as a result of the use of spray equipment with pressure regulators to apply fibrin tissue adhesives. These cases appear to be related to the use of spray equipment at pressures higher than recommended and/or at a very close distance to the tissue surface.
The adverse reactions reported during clinical trials of Artiss and during post-marketing experience with Baxter's fibrin tissue adhesives are described below. The known frequencies of these adverse reactions are based on a controlled clinical trial conducted in 138 patients where ARTISS was used to fix skin grafts in areas without skin due to burns. None of the events observed in the clinical trial were classified as serious.
Table 1 Adverse reactions | |
Adverse reaction | Frequency |
Dermatic cyst | Uncommon |
Pruritus | Common |
Skin graft failure | Common |
Air bubbles in the vascular system (gas embolism) * | Not known |
*There have been cases of gas or air bubbles entering the vascular system (gas embolism) when fibrin sealants are applied with spray equipment using gas or air under pressure; it is believed that the cause of this effect is the improper use of spray equipment (e.g., at pressures higher than recommended and at a very close distance to the tissue surface).
The following adverse reactions have been reported for other fibrin adhesives, but their frequencies cannot be provided: allergy, severe allergic reaction, decreased heart rate, increased heart rate, decreased blood pressure, bleeding, difficulty breathing, discomfort, hives, redness, healing disorders, inflammation, fever, accumulation of lymph and other body fluids under the skin and near the surgical site.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Artiss
- Keep this medicine out of the sight and reach of children.
- Do not use ARTISS after the expiry date stated on the packaging after "EXP".
- Store and transport frozen (at -20°C) without interruption until preparation for application.
- Keep ARTISS in the original packaging to protect it from light.
Storage after thawing:
Unopened thawed bags can be stored for up to 14 days at controlled room temperature (not exceeding +25°C).
Once thawed, do not re-freeze or refrigerate!
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
ARTISS contains two components:
Component 1 = Sealing Protein Solution:
The active ingredients contained in 1 ml of sealing protein solution are:
Human fibrinogen, 91 mg/ml produced from human donor plasma; synthetic aprotinin, 3000 UIC/ml.
The other components are human albumin, L-histidine, niacinamide, polysorbate 80, sodium citrate dihydrate, and water for injectable preparations.
Component 2 = Thrombin Solution
The active ingredients contained in 1 ml of thrombin solution are:
Human thrombin, 4 IU/ml produced from human donor plasma; calcium chloride dihydrate, 40 mmol/ml.
The other components are human albumin, sodium chloride, and water for injectable preparations.
After mixing | 1 ml | 2 ml | 4 ml | 10 ml |
Component 1: Sealing Protein Solution Human fibrinogen (as coagulable protein) Synthetic aprotinin | 45.5 mg 1,500 UIC | 91 mg 3,000 UIC | 182 mg 6,000 UIC | 455 mg 15,000 UIC |
Component 2: Thrombin Solution Human thrombin Calcium chloride dihydrate | 2 IU 20 mmol | 4 IU 40 mmol | 8 IU 80 mmol | 20 IU 200 mmol |
ARTISS contains human factor XIII copurified with human fibrinogen in a range of 0.6 - 5 IU/ml.
Appearance of the Product and Package Contents
Tissue adhesive solutions.
Frozen solutions for tissue adhesive (1 ml, 2 ml, or 5 ml sealing protein solution and 1 ml, 2 ml, or 5 ml thrombin solution in a single-use dual-chamber syringe contained in a bag). Unit package.
Package Contents with PRIMA Syringe:
1 ml, 2 ml, or 5 ml of sealing protein solution and 1 ml, 2 ml, or 5 ml of thrombin solution in a single-use, preloaded, dual-chamber syringe (polypropylene) closed with a screw cap, packaged in two bags, and with a device with 2 union nozzles and 4 application cannulas.
Package Contents with AST Syringe:
1 ml, 2 ml, or 5 ml of sealing protein solution and 1 ml, 2 ml, or 5 ml of thrombin solution in a single-use, preloaded, dual-chamber syringe (polypropylene) closed with a screw cap, packaged in two bags, and with a device with a double syringe plunger, 2 union nozzles, and 4 application cannulas.
The solution is colorless or pale yellow.
Only some package sizes may be marketed.
Marketing Authorization Holder
BAXTER, S.L.
Pouet de Camilo, 2
46394 Ribarroja del Turia (Valencia)
Tel: 962 722 800
Fax: 962 722 795
Manufacturer
Takeda Manufacturing Austria AG
Industriestraße 67
A-1221 Vienna
Austria
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
ARTISSin the following countries: AT, BE, CZ, DE, EL, ES, FI, FR, IE, IT, LU, NL, NO, PL, PT, UK.
Artissin DK, IS, SE.
Date of the last revision of this leaflet: April 2021
Detailed and up-to-date information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
---------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Fertility, Pregnancy, and Lactation
The safety of fibrin sealants/hemostatics has not been established in pregnant or breastfeeding women in controlled clinical trials. No animal studies have been conducted.
Therefore, the product should not be administered to pregnant or breastfeeding women unless it is strictly necessary.
The effects of ARTISS on fertility have not been established.
Posology and Method of Administration
ARTISS is for hospital use only. The use of ARTISS is limited to experienced surgeons who have been properly trained in the use of ARTISS.
Posology
Both the amount of ARTISS to be applied and the frequency of application should always be guided by the underlying clinical needs of the patient.
The dose to be applied depends on several variables, such as the type of surgical intervention, the size of the area, and the method of application, among others.
The physician should individualize the application of the product. In clinical trials, individual doses have normally ranged between 0.2 and 12 ml. Larger volumes may be required in some procedures (e.g., sealing large burned surfaces).
An initial amount of product should be applied to the anatomical area or surface to be treated, sufficient to completely cover the desired application area. If necessary, the application can be repeated in any of the small areas that could not be treated previously. However, reapplying ARTISS to an existing layer of polymerized ARTISS should be avoided, as ARTISS will not adhere to a polymerized layer.
It is recommended that the initial application cover the entire surface area to be treated.
As a guide for surface sealing, 1 package of ARTISS 2 ml (1 ml of sealing protein solution plus 1 ml of thrombin solution) will be sufficient, at a minimum, for an area of 10 cm2.
The skin graft should be placed on the wound bed immediately after the application of ARTISS. The surgeon has up to 60 seconds to manipulate and position the graft before polymerization. After the graft or flap has been positioned, it should be held in the desired position by gentle compression for at least 3 minutes to ensure that ARTISS is fixed properly and that the graft or flap adheres firmly to the underlying tissue.
The amount of Artiss needed depends on the size of the surface to be covered. The approximate surface areas covered by each package size of Artiss when applied by spraying are as follows:
Approximate tissue adhesion area required | Required Artiss package size |
100 cm2 200 cm2 500 cm2 | 2ml 4ml 10ml |
To avoid excessive granulation tissue formation and to ensure the gradual absorption of the solidified fibrin adhesive, only a thin layer of the mixed sealing protein-trombin solutions should be applied.
In clinical trials, ARTISS has not been administered to patients over 65 years of age.
Pediatric Population
Available data are described in section 5.1 of the Summary of Product Characteristics, but a posological recommendation cannot be established.
Method of Administration
For epilesional (topical) use. Do not inject.
For subcutaneous use only. The use of ARTISS in laparoscopic surgery is not recommended.
To ensure safe and optimal use of ARTISS, it should be sprayed using a pressure regulator device that provides a maximum pressure of up to 2.0 bar (28.5 psi).
Before applying ARTISS, the wound surface should be dried using a standard technique (e.g., intermittent application of compresses, swabs, or the use of suction devices). Do not use compressed air or gas to dry the area.
ARTISS should only be sprayed onto visible application areas.
ARTISS should be reconstituted and administered exactly as specified in the instructions and only with the recommended equipment for this product.
For spray application, see the Administration section below.
Before administering ARTISS, precautions should be taken to protect/cover external body parts outside the application area sufficiently to prevent any tissue adhesion in undesired areas.
Special Precautions for Disposal and Other Handling (Final Package: PRIMA Syringe)
General
- Before administering ARTISS, precautions should be taken to cover all external body parts outside the area to be treated to prevent any tissue adhesion in undesired areas.
- To avoid adhesion of ARTISS to gloves and surgical instruments, they should be moistened with a sodium chloride solution before coming into contact with ARTISS.
- As a guide for surface sealing, 1 package of ARTISS 2 ml (1 ml of sealing protein solution plus 1 ml of thrombin solution) will be sufficient, at a minimum, for an area of 10 cm2.
- The required dose depends on the size of the surface to be covered.
- DO NOT apply the two components of ARTISS separately. Both components must be applied together.
- DO NOT expose ARTISS to temperatures above 37°C. DO NOT heat in a microwave.
- DO NOT thaw the product by holding it in your hands.
- DO NOT use ARTISS until it has been completely thawed and warmed to 33°C - 37°C.
- Remove the protective cap from the syringe only when thawing and warming are complete. To facilitate the removal of the syringe cap, rock the cap back and forth and remove the protective cap from the syringe.
- Expel all air from the syringe and then connect the union nozzle and application cannula.
Handling and Preparation Instructions
The inner bag and its contents are sterile unless the integrity of the outer package is compromised. Use a sterile technique to transfer the sterile inner bag and its contents to the sterile field.
The ready-to-use syringe can be thawed and warmed using one of the following methods:
- Rapid thawing/warming (sterile water bath):recommended method
- Thawing/warming in a non-sterile water bath
- Thawing/warming in an incubator
- The ready-to-use syringe can also be thawed and stored at room temperature (not above 25°C) for a maximum of 14 days. It should be warmed before use.
- Rapid thawing/warming (sterile water bath), recommended method:
It is recommended to thaw and warm the two components of the tissue adhesive using a sterile water bath at a temperature of 33°C - 37°C.
- The water bath should not exceed 37°C. To monitor the specified temperature range, the water temperature should be controlled using a thermometer and changed as necessary.
- If the sterile water bath is used for thawing and warming, remove the preloaded syringe from the bags before placing it in the sterile water bath.
Instructions:
Place the inner bag in the sterile field, remove the ready-to-use syringe from the inner bag, and place it directly in the sterile water bath. Ensure that the contents of the ready-to-use syringe are completely submerged in the water.
Table 1: Minimum thawing and warming times with a sterile water bath
Package size | Minimum thawing/warming times 33°C to 37°C, sterile water bath, product removed from bags |
2 ml | 5 minutes |
4 ml | 5 minutes |
10 ml | 10 minutes |
- Thawing/warming in a non-sterile water bath
Instructions:
Leave the ready-to-use syringe in both bags and place it in a non-sterile water bath outside the sterile field for an adequate period (see Table 2). Ensure that the bags remain submerged in the water during the entire thawing time. After thawing, remove the bags from the water bath, dry the outer bag, and place the inner bag with the ready-to-use syringe in the sterile field.
Table 2: Minimum thawing and warming times with a non-sterile water bath
Package size | Minimum thawing/warming times 33°C to 37°C, non-sterile water bath Product in bags |
2 ml | 15 minutes |
4 ml | 20 minutes |
10 ml | 35 minutes |
- Thawing/warming in an incubator
Instructions:
Leave the ready-to-use syringe in both bags and place it in an incubator outside the sterile field for an adequate period (see Table 3). After thawing/warming, remove the bags from the incubator, remove the outer bag, and place the inner bag with the ready-to-use syringe in the sterile field.
Table 3: Minimum thawing and warming times in an incubator
Package size | Minimum thawing/warming times 33°C to 37°C, incubator Product in bags |
2 ml | 40 minutes |
4 ml | 50 minutes |
10 ml | 90 minutes |
- Thawing at room temperature (not above +25°C) BEFORE warming:
Instructions:
Leave the ready-to-use syringe in both bags and thaw it at room temperature outside the sterile field for an adequate period (see Table 4). Once thawed, to warm the product for use, warm it in the outer bag in an incubator. After thawing at room temperature, the maximum time the product can be stored (in both bags) at room temperature is 14 days.
Table 4: Minimum thawing times at room temperature (= TA) outside the sterile field and additional warming times in an incubator at 33°C to 37°C
Package size | Minimum thawing times of the product at room temperature (not above 25°C) followed by additional warming in an incubator at 33°C to 37°C Product in bags |
Thawing at room temperature (not above 25°C) | Warming in incubator (33°C-37°C) |
2 ml | 80 minutes + 11 minutes |
4 ml | 90 minutes + 13 minutes |
10 ml | 160 minutes + 25 minutes |
Stability after Thawing
After thawing and warming(at temperatures between 33°C and 37°C, methods 1, 2, and 3), chemical and physical stability has been demonstrated for 4 hours at a temperature between 33°C and 37°C.
In the case of the thawedproduct at room temperature in the unopened bag (method 4), chemical and physical stability has been demonstrated for 14 days at temperatures not above 25°C. Warm to a temperature between 33°C and 37°C immediately before use.
From a microbiological point of view, unless the opening and thawing method excludes the risk of microbial contamination, the product should be used immediately after being warmed to 33°C - 37°C.
If not used immediately, the conditions and times of storage necessary for its use are the responsibility of the user.
Do not refreeze or refrigerate once thawing has begun.
Handling after Thawing/Before Application
To achieve optimal mixing of the two solutions and optimal solidification of the fibrin tissue adhesive, keep the two components at 33°C - 37°C until application.
The sealing protein solution and thrombin solution should be transparent or slightly opalescent. Do not use solutions that are turbid or have deposits. The thawed product should be visually inspected before use to rule out the presence of particles and discoloration or any variation in appearance. If any of these circumstances occur, discard the solutions.
The thawed sealing protein solution should be a slightly viscous liquid. If the solution has the consistency of a solidified gel, it should be assumed that it has denatured (possibly due to interruption of the cold chain or excessive heat during warming). In this case, DO NOT use ARTISS in any way.
- Remove the syringe from the bags shortly before use.
- Use ARTISS only when it has been completely thawed and warmed (liquid consistency).
- Remove the protective cap from the syringe immediately before application.
In the case of the PRIMA syringe: To facilitate the removal of the syringe cap, rock the cap back and forth and remove the protective cap from the syringe.
Administration without Spraying with the PRIMA Syringe:
For application, the ready-to-use dual-chamber syringe with the sealing protein solution and thrombin solution must be connected to a union nozzle and an application cannula, included in the application device kit. The piece that connects the syringe plungers ensures that equal volumes of the two components of the tissue adhesive will exit through the union nozzle towards the application cannula, where they will mix before application.
PRIMA Syringe Instructions:
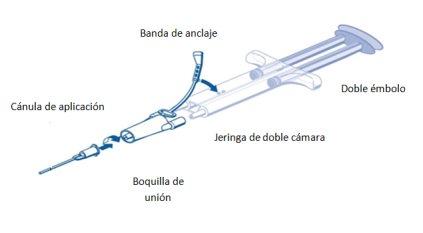
- Expel all air from the syringe before connecting it to any application device.
- Align the union nozzle and connect it to the application cannula.
- Align the union nozzle and anchor the lateral of the syringe in the opening of the anchor band.
- Connect the nozzles of the ready-to-use double-chamber syringe to the union nozzle and ensure that both are firmly attached.
- Secure the union nozzle by fixing the anchor band to the ready-to-use double-chamber syringe.
- If the anchor band breaks, use the union nozzle included in the equipment.
- If no spare union nozzle is available, it is still possible to use the system if care is taken to ensure that the connection is secure and leak-proof.
- DO NOT expel the air remaining in the union nozzle.
- Connect an application cannula to the union nozzle.
- DO NOT expel the air remaining in the union nozzle and within the application cannula until application starts, as this could obstruct the application cannula.
Administration
Before applying ARTISS, it is necessary to dry the wound surface using a standard technique (e.g., intermittent application of compresses, swabs, use of suction devices). Do not use compressed air or gas to dry the area.
- Apply the mixture of sealant protein-thrombin solution over the surface or surfaces of the parts to be sealed by slowly pressing the rear of the common plunger.
- In surgical procedures that require the use of minimal volumes of fibrin tissue adhesive, it is recommended to expel and discard the first drops of the product.
- After applying ARTISS, wait at least 3 minutes to achieve sufficient polymerization.
Note: If the application of the fibrin tissue adhesive components is interrupted, clots may form in the cannula. In this case, replace the application cannula with a new one immediately before resuming application. If the openings of the union nozzle become clogged, use the additional union nozzle provided in the package.
BAXTER also provides other accessories for application that are particularly suitable for, e.g., application in large or hard-to-reach areas. When using these application devices, the Instructions for Use of the devices must be strictly followed.
For further preparation instructions, consult the responsible nurse or physician.
Spray Application
The pressure regulator must be used according to the manufacturer's instructions.
When applying ARTISS with a spray device, ensure that the pressure and distance to the tissue are within the intervals recommended by the manufacturer as follows:
Pressure, distance, and equipment recommended for ARTISS spray application | |||||
Spray equipment to be used | Application tips to be used | Pressure regulator to be used | Recommended target tissue distance | Recommended spray pressure | |
Surgery with open wound of subcutaneous tissue | Tisseel/Artiss spray equipment | n.a. | EasySpray | 10 – 15 cm | 1.5-2.0 bar (21.5-28.5 psi) |
Tisseel/Artiss spray equipment, 10-pack | n.a. | EasySpray |
When spraying ARTISS, monitor changes in arterial pressure, pulse, oxygen saturation, and CO2level at the end of expiration to detect possible gas embolism (air or gas) (see sections 4.2 and 4.4 of the Technical Data Sheet).
When using accessory nozzles with this product, follow the instructions for use of the nozzles.
Disposal
Disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
Special disposal and handling precautions (final packaging: AST syringe)
General
- Before administering ARTISS, take precautions to cover all external body parts outside the treatment area to prevent any tissue adhesion in unwanted areas.
- To avoid adhesion of ARTISS to gloves and surgical instruments, these should be moistened with a sodium chloride solution before contact occurs.
- As a guide for surface sealing, 1 package of ARTISS (2 ml, 1 ml sealant protein solution + 1 ml thrombin solution) will be sufficient for an area of at least 10 cm2.
- The required dose depends on the size of the surface to be covered.
- DO NOT apply the two components of ARTISS separately. Both components must be applied together.
- DO NOT expose ARTISS to temperatures above 37°C. DO NOT heat in a microwave.
- DO NOT thaw the product by holding it in your hands.
- DO NOT use ARTISS until it has been completely thawed and warmed to 33°C – 37°C.
- Remove the protective cap from the syringe only when thawing and warming are complete.
- Expel all air from the syringe and then connect the union nozzle and application cannula.
Handling and preparation instructions
The inner bag and its contents are sterile unless the integrity of the outer packaging is compromised. Use a sterile technique to transfer the sterile inner bag and its contents to the sterile field.
The ready-to-use syringe can be thawed and warmed using one of the following methods:
- Rapid thawing/warming (sterile water bath),recommended method:
- Thawing/warming in a non-sterile water bath
- Thawing/warming in an incubator
- The ready-to-use syringe can also be thawed and stored at room temperature (not above 25°C) for a maximum of 14 days. It must be warmed before use.
- Rapid thawing/warming (sterile water bath), recommended method:
It is recommended to thaw and warm the two components of the fibrin tissue adhesive using a sterile water bath at a temperature of 33°C-37°C.
- The water bath should not exceed 37°C. To monitor the specified temperature range, the water temperature should be controlled using a thermometer and changed as necessary.
- If the sterile water bath is used for thawing and warming, remove the pre-filled syringe from the bags before placing it in the sterile water bath.
Instructions:
Place the inner bag in the sterile field, remove the ready-to-use syringe from the inner bag, and place it directly in the sterile water bath. Ensure that the contents of the ready-to-use syringe are completely submerged in the water.
Table 1: Minimum thawing and warming times with a sterile water bath
Package size | Minimum thawing/warming times 33°C to 37°C, sterile water bath, Product removed from bags |
2 ml | 5 minutes |
4 ml | 5 minutes |
10 ml | 12 minutes |
- Thawing/warming in a non-sterile water bath
Instructions:
Leave the ready-to-use syringe in both bags and place it in a non-sterile water bath outside the sterile field for an appropriate period (see Table 2). Ensure that the bags remain submerged in the water for the entire thawing time. After thawing, remove the bags from the water bath, dry the outer bag, and place the inner bag with the ready-to-use syringe in the sterile field.
Table 2: Minimum thawing and warming times with a non-sterile water bath
Package size | Minimum thawing/warming times 33°C to 37°C, non-sterile water bath Product in bags |
2 ml | 30 minutes |
4 ml | 40 minutes |
10 ml | 80 minutes |
- Thawing/warming in an incubator
Instructions:
Leave the ready-to-use syringe in both bags and place it in an incubator outside the sterile field for an appropriate period (see Table 3). After thawing/warming, remove the bags from the incubator, remove the outer bag, and place the inner bag with the ready-to-use syringe in the sterile field.
Table 3: Minimum thawing and warming times in an incubator
Package size | Minimum thawing/warming times 33°C to 37°C, incubator Product in bags |
2 ml | 40 minutes |
4 ml | 85 minutes |
10 ml | 105 minutes |
- Thawing at room temperature (not above +25°C) BEFORE warming:
Instructions:
Leave the ready-to-use syringe in both bags and thaw it at room temperature outside the sterile field for an appropriate period (see Table 4). Once thawed, to warm the product for use, warm it in the outer bag in an incubator. After thawing at room temperature, the maximum time the product can be stored (in both bags) at room temperature is 14 days.
Table 4: Minimum thawing times at room temperature (= RT) outside the sterile field and additional warming times in an incubator at 33°C to 37°C
Package size | Minimum thawing times of the product at room temperature (not above 25°C) followed by additional warming in an incubator at 33°C to 37°C Product in bags |
Thawing at room temperature (not above 25°C) | Warming in incubator (33°C - 37°C) |
2 ml | 60 minutes + 15 minutes |
4 ml | 110 minutes + 25 minutes |
10 ml | 160 minutes + 35 minutes |
Stability after thawing
After thawing and warming(at temperatures between 33°C and 37°C, methods 1, 2, and 3), chemical and physical stability has been demonstrated for 4 hours at a temperature between 33°C and 37°C.
In the case of the product thawedat room temperature in the unopened bag (method 4), chemical and physical stability has been demonstrated for 14 days at temperatures not above 25°C. Warm to a temperature between 33°C and 37°C immediately before use.
From a microbiological point of view, unless the opening and thawing method excludes the risk of microbial contamination, the product must be used immediately after being warmed to between 33°C and 37°C.
If not used immediately, the conditions and times of storage necessary for its use are the responsibility of the user.
Do not re-freeze or refrigerate once thawing has begun.
Handling after thawing/before application
To achieve optimal mixing of the two solutions and optimal solidification of the fibrin tissue adhesive, keep the two components at 33°C-37°C until application.
The sealant protein and thrombin solutions should be clear or slightly opalescent. Do not use solutions that are cloudy or have deposits. The thawed product must be visually inspected before use to rule out the presence of particles and discoloration or any variation in appearance. If any of these circumstances occur, discard the solutions.
The thawed sealant protein solution should be a slightly viscous liquid. If the solution has the consistency of a solidified gel, it should be assumed that it has denatured (possibly due to interruption of the cold chain or excessive heat during warming). In this case, DO NOT use ARTISS in any way.
- Remove the syringe from the bags shortly before use.
- Use ARTISS only when it has been completely thawed and warmed (liquid consistency).
- Remove the protective cap from the syringe immediately before application.
Administration without spraying with the AST syringe:
For application, the ready-to-use double-chamber syringe with sealant protein solution and thrombin solution must be connected to a union nozzle and an application cannula, included in the application device kit. The common plunger of the ready-to-use double-chamber syringe, included in the application device kit, ensures that equal volumes of the two components of the fibrin tissue adhesive are expelled through a union nozzle to the application cannula, where they will mix before application.
AST syringe usage instructions:
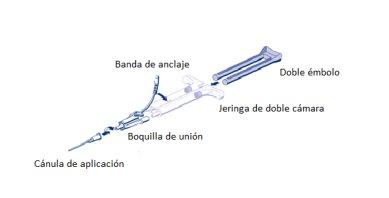
- Expel all air from the syringe before connecting it to any application device.
- Align the union nozzle and anchor the lateral of the syringe in the opening of the anchor band.
- Connect the nozzles of the ready-to-use double-chamber syringe to the union nozzle and ensure that both are firmly attached.
- Secure the union nozzle by fixing the anchor band to the ready-to-use double-chamber syringe.
- If the anchor band breaks, use the union nozzle included in the equipment.
- If no spare union nozzle is available, it is still possible to use the system if care is taken to ensure that the connection is secure and leak-proof.
- DO NOT expel the air remaining in the union nozzle.
- Connect an application cannula to the union nozzle.
- DO NOT expel the air remaining in the union nozzle and within the application cannula until application starts, as this could obstruct the application cannula.
Administration
Before applying ARTISS, it is necessary to dry the wound surface using a standard technique (e.g., intermittent application of compresses, swabs, use of suction devices). Do not use compressed air or gas to dry the area.
- Apply the mixture of sealant protein-thrombin solution over the surface or surfaces of the parts to be sealed by slowly pressing the rear of the common plunger.
- In surgical procedures that require the use of minimal volumes of fibrin tissue adhesive, it is recommended to expel and discard the first drops of the product.
- After applying ARTISS, wait at least 3 minutes to achieve sufficient polymerization.
Note: If the application of the fibrin tissue adhesive components is interrupted, clots may form in the cannula. In this case, replace the application cannula with a new one immediately before resuming application. If the openings of the union nozzle become clogged, use the additional union nozzle provided in the package.
BAXTER also provides other accessories for application that are particularly suitable for, e.g., application in large or hard-to-reach areas. When using these application devices, the Instructions for Use of the devices must be strictly followed.
For further preparation instructions, consult the responsible nurse or physician.
Spray Application
The pressure regulator must be used according to the manufacturer's instructions.
When applying ARTISS with a spray device, ensure that the pressure and distance to the tissue are within the intervals recommended by the manufacturer as follows:
Pressure, distance, and equipment recommended for ARTISS spray application | |||||
Spray equipment to be used | Application tips to be used | Pressure regulator to be used | Recommended target tissue distance | Recommended spray pressure | |
Surgery with open wound of subcutaneous tissue | Tisseel/Artiss spray equipment | n.a. | EasySpray | 10 – 15 cm | 1.5-2.0 bar (21.5-28.5 psi) |
Tisseel/Artiss spray equipment, 10-pack | n.a. | EasySpray |
When spraying ARTISS, monitor changes in arterial pressure, pulse, oxygen saturation, and CO2level at the end of expiration to detect possible gas embolism (air or gas) (see sections 4.2 and 4.4 of the Technical Data Sheet).
When using accessory nozzles with this product, follow the instructions for use of the nozzles.
Disposal
Disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ARTISS Tissue Adhesive Solution, UltracoldDosage form: TISSUE ADHESIVE, 50-90 mg/ml 800-1200 IUActive substance: combinationsManufacturer: Omrix BiopharmaceuticalsPrescription requiredDosage form: TISSUE ADHESIVE, -Active substance: combinationsManufacturer: Corza Medical GmbhPrescription requiredDosage form: TISSUE ADHESIVE, -Active substance: combinationsManufacturer: Corza Medical GmbhPrescription required
Online doctors for ARTISS Tissue Adhesive Solution, Ultracold
Discuss questions about ARTISS Tissue Adhesive Solution, Ultracold, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















