VEDROP 50 mg/ml ORAL SOLUTION

How to use VEDROP 50 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Vedrop 50 mg/ml Oral Solution
Tocofersolan
This medicine is subject to additional monitoring, which will allow quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet:
- What is Vedrop and what is it used for
- What you need to know before you take Vedrop
- How to take Vedrop
- Possible side effects
- Storage of Vedrop
- Contents of the pack and other information
1. What is Vedrop and what is it used for
Vedrop contains vitamin E (in the form of tocofersolan). This medicine is used to treat vitamin E deficiency caused by impaired digestive absorption (where nutrients from food are not easily absorbed during digestion) in patients from birth (term newborns) to 18 years of age with chronic cholestasis (a hereditary or congenital disease in which bile cannot pass from the liver to the intestine).
2. What you need to know before you take Vedrop
Do not take Vedrop
- If you are allergic to vitamin E (d-alpha-tocopherol) or to any of the other ingredients of this medicine (listed in section 6).
- Vedrop should not be administered to premature babies.
Warnings and precautions
Consult your doctor before starting Vedrop if you have:
- Kidney problems or dehydration. Vedrop should be used with caution and kidney function should be carefully monitored, as the polyethylene glycol, which is part of the active ingredient tocofersolan, can damage the kidneys.
- Liver problems. Vedrop should be used with caution and liver function should be strictly monitored.
Taking Vedrop with other medicines
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Tell your doctor or pharmacist if you are taking:
- Certain medicines that reduce blood viscosity (oral anticoagulants such as warfarin). Your doctor will ask you to undergo regular blood tests and may adjust the dose to avoid a higher risk of bleeding.
- Fat-soluble vitamins (such as vitamins A, D, E, or K) or highly fat-soluble medicines (such as corticosteroids, cyclosporine, tacrolimus, antihistamines). Vedrop may increase their absorption during digestion, so the doctor will monitor the effect of the treatment and adjust the dose when necessary.
Pregnancy and breastfeeding
There are no clinical data available on the use of this medicine during pregnancy. Inform your doctor if you are pregnant so that he/she can decide whether to use the medicine.
There are no data on whether this medicine is excreted in breast milk. Inform your doctor if you want to breastfeed your child so that he/she can decide whether to use the medicine. Your doctor will help you make the best decision for you and your child.
Consult your doctor or pharmacist before taking any medicine.
Driving and using machines
It is unlikely that Vedrop will affect your ability to drive and use machines.
Vedrop contains methyl-parahydroxybenzoate sodium (E219) and ethyl-parahydroxybenzoate sodium (E215), which may cause allergic reactions (possibly delayed).
Vedrop contains 0.18 mmol (4.1 mg) of sodium per ml. Consult your doctor if you are on a low-sodium diet.
3. How to take Vedrop
Follow exactly the administration instructions of this medicine given by your doctor. In case of doubt, consult your doctor or pharmacist again.
The usual dose is 0.34 ml/kg/day.
Your doctor will prescribe the dose in ml.
Your doctor will adjust the dose of this medicine according to your blood vitamin E levels.
Method of administration
Swallow the solution with or without water. Use it only with the oral syringe included in the package.
You can take Vedrop before or during meals, with or without water.
To measure the dose:
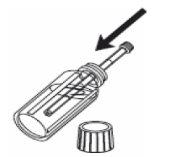
1- Open the bottle. 2- Insert the oral syringe into the bottle. |
|
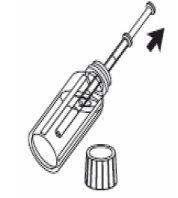
3- Fill the oral syringe with the liquid by pulling the plunger to the mark corresponding to the amount in milliliters (ml) prescribed by your doctor. |
|
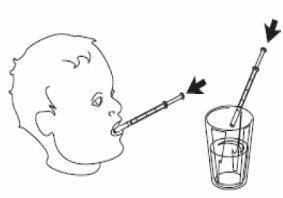
4- Remove the oral syringe from the bottle. 5- Empty the contents of the syringe by pressing the plunger to the bottom, either:
or
|
|
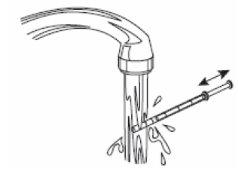
6- Close the bottle. 7- Wash the syringe with water. |
|
If you take more Vedrop than you should
If you take high doses of vitamin E, you may experience transient diarrhea and stomach pain. Consult your doctor or pharmacist if the symptoms persist for more than two days.
If you forget to take Vedrop
Do not take the missed dose and return to the regular scheduled administration. Do not take a double dose to make up for the missed doses.
If you stop taking Vedrop
Do not stop treatment without consulting your doctor, as it may cause a vitamin E deficiency that affects your health. Contact your doctor or pharmacist before stopping treatment.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported:
Common side effects (may affect up to 1 in 10 people)
- Diarrhea
Uncommon side effects (may affect up to 1 in 100 people)
- Asthenia (feeling of weakness)
- Headache
- Hair loss
- Itching
- Rash
- Abnormal sodium levels in the blood
- Abnormal potassium levels in the blood
- Increased transaminases (liver enzymes)
Frequency not known(cannot be estimated from the available data)
- Stomach pain
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vedrop
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the package and on the bottle after EXP. The expiry date is the last day of the month stated.
- This medicine does not require any special storage conditions.
- Discard the medicine one month after it has been opened, even if some solution remains.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Vedrop
- The active substance is tocofersolan. Each ml of solution contains 50 mg of d-alpha-tocopherol in the form of tocofersolan, equivalent to 74.5 IU of tocopherol.
- The other ingredients are: potassium sorbate, methyl-parahydroxybenzoate sodium (E219) and ethyl-parahydroxybenzoate sodium (E215) (see the end of section 2 for more information on these two excipients), glycerol, disodium phosphate dodecahydrate, concentrated hydrochloric acid, purified water.
Appearance of Vedrop and pack contents
Vedrop is a pale yellow, slightly viscous oral solution contained in a brown glass bottle, sealed with a safety cap. The bottles contain 10 ml, 20 ml, or 60 ml of oral solution. Each pack contains one bottle and one oral syringe (a 1 ml syringe with a 10 ml or 20 ml bottle, a 2 ml syringe with a 60 ml bottle).
Marketing authorisation holder
Recordati Rare Diseases
Immeuble “Le Wilson”
70, avenue du General de Gaulle
F-92800 Puteaux
France
Manufacturer
Recordati Rare Diseases
Immeuble “Le Wilson”
70, avenue du Général de Gaulle
92800 Puteaux
France
or
Recordati Rare Diseases
Eco River Parc
30, rue des Peupliers
F-92000 Nanterre
France
You can obtain further information on this medicine by contacting the local representative of the marketing authorisation holder.
Belgium/België/Belgien Recordati Tel: +32 2 46101 36 | Lithuania Recordati AB. Tel: + 46 8 545 80 230 Sweden |
Bulgaria Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Luxembourg/Luxemburg Recordati Tel: +32 2 46101 36 Belgium |
Czech Republic Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Hungary Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Denmark Recordati AB. Tel: +46 8 545 80 230 Sweden | Malta Recordati Rare Diseases Tel: +33 1 47 73 64 58 France |
Germany Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 | Netherlands Recordati Tel: +32 2 46101 36 Belgium |
Estonia Recordati AB. Tel: + 46 8 545 80 230 Sweden | Norway Recordati AB. Tel: +46 8 545 80 230 Sweden |
Greece Recordati Rare Diseases Tel: +33 1 47 73 64 58 France | Austria Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 Germany |
Spain Recordati Rare Diseases Spain S.L.U. Tel: + 34 91 659 28 90 | Poland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
France Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 | Portugal Jaba Recordati S.A. Tel: +351 21 432 95 00 |
Croatia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Romania Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Ireland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Slovenia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Iceland Recordati AB. Tel: +46 8 545 80 230 Sweden | Slovakia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Italy Recordati Rare Diseases Italy Srl Tel: +39 02 487 87 173 | Finland Recordati AB. Tel: +46 8 545 80 230 Sweden |
Cyprus Recordati Rare Diseases Tel: +33 1 47 73 64 58 France | Sweden Recordati AB. Tel: +46 8 545 80 230 |
Latvia Recordati AB. Tel: + 46 8 545 80 230 Sweden | United Kingdom Recordati Rare Diseases UK Ltd. Tel: +44 (0)1491 414333 |
Date of last revision of this leaflet:
This medicine has been authorised under exceptional circumstances. This means that due to the rarity of the disease, it has not been possible to obtain complete information on this medicine.
The European Medicines Agency will review any new information that may become available every year and this leaflet will be updated as necessary.
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VEDROP 50 mg/ml ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 50 mg/mlActive substance: tocofersolanManufacturer: Recordati Rare DiseasesPrescription requiredDosage form: CAPSULE, 2000 mgActive substance: tocopherol (vit E)Manufacturer: Chiesi España S.A.U.Prescription requiredDosage form: CAPSULE, 400 mgActive substance: tocopherol (vit E)Manufacturer: Chiesi España S.A.U.Prescription required
Online doctors for VEDROP 50 mg/ml ORAL SOLUTION
Discuss questions about VEDROP 50 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions