
VAZKEPA 998 mg SOFT GEL CAPSULES

How to use VAZKEPA 998 mg SOFT GEL CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Vazkepa 998 mg Soft Capsules
icosapent ethyl
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Vazkepa and what is it used for
- What you need to know before you take Vazkepa
- How to take Vazkepa
- Possible side effects
- Storage of Vazkepa
- Contents of the pack and other information
1. What is Vazkepa and what is it used for
Vazkepa contains the active substance icosapent ethyl, a highly purified omega-3 fatty acid derived from fish oil.
Vazkepa reduces the levels of triglycerides (a type of fat) in the blood and is used with a statin (which reduces cholesterol in the blood) to prevent cardiovascular events such as:
- heart attack
- stroke
- death from heart disease or vascular disease
Vazkepa is used in adults with high levels of triglycerides in the blood, who already have heart disease or diabetes, and other conditions that increase the risk of cardiovascular events.
2. What you need to know before you take Vazkepa
Do not take Vazkepa
- if you are allergic to icosapent ethyl, soy or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before taking Vazkepa:
- If you are allergic to fish or shellfish.
- If you have liver problems.
- If you have an irregular heartbeat(atrial fibrillation or flutter).
- If you are taking an anticoagulant (which prevents blood clotting), medicines that inhibit platelets in the blood or have a risk of bleeding.
Talk to your doctor if any of the above applies to you.
Blood tests
During treatment, your doctor will perform regular blood tests to check for liver problems and to check the clotting of your blood.
Children and adolescents
This medicine should not be given to children or young people under 18 years of age because it has not been studied in these individuals.
Other medicines and Vazkepa
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
If you are taking other medicines at the same time as Vazkepa that affect blood clotting, such as an anticoagulant, you will have blood tests during treatment.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Vazkepa should not be used during pregnancy unless your doctor advises you to take it.
Breastfeeding
Vazkepa should not be used during breastfeeding, because its effect on the newborn is unknown. Your doctor will help you weigh the benefits of treatment against any risk to your breastfed baby.
Fertility
Talk to your doctor about fertility during treatment.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use tools or machines.
Vazkepa contains maltitol, sorbitol and soy lecithin
Maltitol (E965)
If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
Sorbitol (E420)
This medicine contains 83 mg of sorbitol in each capsule.
Sorbitol is a source of fructose. If your doctor has told you that you have an intolerance to some sugars, or you have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disorder, in which the patient cannot break down fructose which can cause serious side effects, talk to your doctor before taking this medicine.
Soy lecithin
This medicine contains soy lecithin. Do not use this medicine if you are allergic to peanuts or soy.
3. How to take Vazkepa
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, ask your doctor or pharmacist again. Do not change the dose without consulting your doctor.
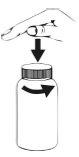
How to open the bottle
Press the cap down and turn it in the opposite direction to the arrows.
How much to take
The recommended dose is two capsules by mouth, twice a day, with or after a meal.
Swallow the capsules whole; do not break, crush, dissolve or chew them.
Use in elderly patients
No dose adjustment is necessary in elderly patients. They can take the recommended dose as usual.
If you take more Vazkepa than you should
If you accidentally take more capsules than your doctor has prescribed, talk to your doctor or pharmacist.
If you forget to take Vazkepa
If you forget a dose, take it as soon as you remember. However, if you forget to take the medicine for the whole day, just take the next scheduled dose. Do not take a double doseto make up for the forgotten dose. If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
If you stop taking Vazkepa
Do not stop taking this medicine until you have talked to your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Talk to your doctor
- if you have heart palpitations or an irregular heartbeat. These could be symptoms of a serious condition called atrial fibrillation. This is a common side effect (may affect up to 1 in 10 people):
- if you bruise easily or do not stop bleeding. This is a very common side effect (may affect more than 1 in 10 people). Your risk of bleeding may increase if you are also taking an anticoagulant.
Seek medical helpif you experience any of the following side effects. These symptoms may be due to a serious condition called hypersensitivitythat can occur at any time during treatment. This is an uncommon side effect (may affect up to 1 in 100 people):
- difficulty breathing
- throat tightness or itching
- swelling of the lips
- hives (red patches on the skin) rash and itching of the skin
- stomach pain or colic
- diarrhea
- nausea and vomiting
Other side effects that may occur
Common side effects (may affect up to 1 in 10 people):
- swelling of the hands, arms, legs and feet
- pain in the muscles, bones or joints
- gout (painful swelling of the joints due to uric acid accumulation)
- skin rash
- constipation
- belching
Uncommon side effect (may affect up to 1 in 100 people)
- unpleasant taste
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vazkepa
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the bottle or on the carton of the blisters after EXP. The expiry date refers to the last day of the month shown.
Store below 30°C.
Bottle: keep the bottle tightly closed to protect from moisture.
Blister: store in the original package to protect from moisture.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Vazkepa
- The active substanceis icosapent ethyl. Each Vazkepa capsule contains 998 mg of icosapent ethyl.
- The other ingredients are
- all-rac-alpha-tocopherol, gelatin, glycerol, maltitol liquid (E965), sorbitol liquid (non-crystallizing) (E420), purified water and soy lecithin (see section 2 "Vazkepa contains maltitol, sorbitol and soy lecithin").
- printing ink: titanium dioxide, propylene glycol, hypromellose.
Appearance of the product and pack contents
In this pack, you will find soft, oblong capsules, 25 x 10 mm, with "IPE" printed in white ink, with a light yellow to amber-colored coating that contains a clear to pale yellow liquid.
The bottles containing 120 capsules are white, 300 ml, high-density polyethylene (HDPE) with a polypropylene child-resistant closure. Pack size of one bottle or three bottles per carton.
The blister packs contain 4x2 capsules in unit-dose, perforated PVC/PCTFE/Al blisters.
Marketing authorisation holder
Amarin Pharmaceuticals Ireland Limited
88 Harcourt Street
Dublin 2, D02DK18
Ireland
Manufacturer
MIAS Pharma Limited
Suite 2, Stafford House, Strand Road
Portmarnock
Co. Dublin
Ireland
You can request more information about this medicine from the local representative of the marketing authorisation holder:
Belgium Amarin Pharmaceuticals Ireland Limited Tel: 0800-75394 | Lithuania Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Greece Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Luxembourg Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Czech Republic Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Hungary Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Denmark Amarin Pharmaceuticals Ireland Limited Tel: +46-84-4685033 | Malta Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Germany Amarin Germany GmbH Tel: 0800-0008975 | Netherlands Amarin Pharmaceuticals Ireland Limited Tel: 0800-0228734 |
Estonia Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Norway Amarin Pharmaceuticals Ireland Limited Tel: +46-84-4685033 |
Greece Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Austria Amarin Pharmaceuticals Ireland Limited Tel: 0800-281516 |
Spain Amarin Pharmaceuticals Ireland Limited Tel: 900806101 | Poland Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
France Amarin Pharmaceuticals Ireland Limited Tel: 0800-991006 | Portugal Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Croatia Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Romania Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Ireland Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Slovenia Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Iceland Amarin Pharmaceuticals Ireland Limited Tel: +46-84-4685033 | Slovakia Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 |
Italy Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Finland Amarin Pharmaceuticals Ireland Limited Tel: +46-84-4685033 |
Cyprus Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | Sweden Amarin Pharmaceuticals Ireland Limited Tel: +46-84-4685033 |
Latvia Amarin Pharmaceuticals Ireland Limited Tel: +353(0)16915000 | United Kingdom (Northern Ireland) Amarin Pharmaceuticals Ireland Limited Tel: 0800-0478673 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price205.83 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VAZKEPA 998 mg SOFT GEL CAPSULESDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Kern Pharma S.L.Prescription requiredDosage form: CAPSULE, 1000 mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Strides Pharma (Cyprus) LimitedPrescription requiredDosage form: CAPSULE, 1000mgActive substance: omega-3-triglycerides incl. other esters and acidsManufacturer: Tarbis Farma S.L.Prescription required
Online doctors for VAZKEPA 998 mg SOFT GEL CAPSULES
Discuss questions about VAZKEPA 998 mg SOFT GEL CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











