TYENNE 162 mg INJECTABLE SOLUTION IN PRE-FILLED PEN

How to use TYENNE 162 mg INJECTABLE SOLUTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Tyenne 162 mg solution for injection in pre-filled pen
tocilizumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
In addition to this leaflet, you will be given a Patient Information Card, which contains important safety information that you should know before you receive and during treatment with Tyenne.
Contents of the pack
- What is Tyenne and what is it used for
- What you need to know before you use Tyenne
- How to use Tyenne
- Possible side effects
- Storage of Tyenne
- Contents of the pack and other information
1. What is Tyenne and what is it used for
Tyenne contains the active substance tocilizumab, which is a protein obtained from specific immune cells (monoclonal antibody), that blocks the action of a specific type of protein (cytokine) called interleukin-6. This protein is involved in inflammatory processes in the body, and blocking it can reduce inflammation. Tyenne is indicated for the treatment of:
- adults with moderate to severe active rheumatoid arthritis (RA), which is an autoimmune disease, if previous treatments have not worked well.
- adults with severe, active, and progressive rheumatoid arthritis (RA), who have not been previously treated with methotrexate.
Tyenne helps to reduce the symptoms of RA such as pain and swelling in the joints and can also improve performance in daily tasks. Tyenne has been shown to slow down the progression of damage to the cartilage and bones of the joints caused by the disease and improve the ability to perform daily activities.
Tyenne is normally used in combination with another medicine for RA called methotrexate. However, Tyenne may be given alone if your doctor decides that methotrexate is not suitable.
- adults with a disease of the arteries called giant cell arteritis (GCA), caused by inflammation of the largest arteries in the body, especially those that supply blood to the head and neck. Symptoms may include headache, fatigue, and jaw pain. Effects may include stroke and blindness.
Tyenne may reduce the pain and swelling of the arteries and veins in the head, neck, and arms.
GCA is often treated with medicines called steroids. These are usually effective, but can have side effects if used at high doses for a long time. Reducing the dose of steroids can also lead to a flare-up of GCA. Adding Tyenne to treatment can make the time of use of steroids shorter, while still controlling the disease.
- children and adolescents, 12 years of age and older, with active systemic juvenile idiopathic arthritis (sJIA), an inflammatory disease that causes pain and swelling in one or more joints, as well as fever and rash.
Tyenne is used to improve the symptoms of sJIA. It can be given in combination with methotrexate or alone.
- children and adolescents, 12 years of age and older, with active polyarticular juvenile idiopathic arthritis (pJIA). This is an inflammatory disease that causes pain and swelling in one or more joints.
Tyenne is used to improve the symptoms of pJIA. It can be given in combination with methotrexate or alone.
2. What you need to know before you use Tyenne
Tyenne will not be given to you
- If you are allergicto tocilizumab or any of the other ingredients of this medicine (listed in section 6).
- If you have a severe active infection.
If any of these apply to you, talk to your doctor or nurse.
Warnings and precautions
Talk to your doctor or nurse before you start receiving Tyenne.
- If you experience allergic reactionssuch as chest tightness, wheezing, dizziness, or severe dizziness, swelling of the lips, or rash during or after the infusion, tell your doctor immediately.
- If you have any type of infection, either short-term or long-term, or if you get infections often tell your doctor immediatelyif you feel unwell. Tyenne may reduce the ability of your body to respond to infections and may make an existing infection worse or increase the likelihood of getting a new infection.
- If you have had tuberculosis, tell your doctor. Your doctor will check for signs and symptoms of tuberculosis before starting treatment with Tyenne. Tell your doctor immediately if symptoms of tuberculosis (persistent cough, weight loss, general malaise, low-grade fever), or any other infection appear during or after treatment.
- If you have had intestinal ulcer or diverticulitis, tell your doctor. Symptoms would include abdominal pain and unexplained changes in bowel habits with fever.
- If you have liver disease, tell your doctor. Before using Tyenne, your doctor will perform a blood test to measure your liver function.
- If a patient has been recently vaccinated(adult or child) or is scheduled to be vaccinated, tell your doctor. All patients, especially children, must be up to date with their vaccination schedule before starting treatment with Tyenne, unless urgent treatment is required. Certain types of vaccines must not be given while receiving Tyenne.
- If you have cancer, tell your doctor. Your doctor will have to decide if you can continue treatment with Tyenne.
- If you have cardiovascular risk factors, such as high blood pressure, high cholesterol levels, tell your doctor. These factors need to be controlled while receiving treatment with Tyenne.
If you have moderate to severe kidney problems, your doctor will monitor you.
- If you have persistent headaches.
Your doctor will perform blood tests before you receive Tyenne and during treatment to determine if you have a low white blood cell count, low platelet count, or elevated liver enzymes.
Children and adolescents
Tyenne is not recommended for use in children under 2 years of age.
Tell your doctor if the child has a history of macrophage activation syndrome(uncontrolled activation and proliferation of specific blood cells). Your doctor will decide if the child can continue receiving Tyenne.
Other medicines and Tyenne
Tell your doctor if you are taking, have recently taken, or might take any other medicines (or if the patient you are caring for is taking them). This includes medicines obtained without a prescription. Tyenne may affect how some medicines work, and a dose adjustment may be needed. Tell your doctorif you are using medicines that contain any of these active substances:
- methylprednisolone, dexamethasone, used to reduce inflammation,
- simvastatin or atorvastatin, used to reduce cholesterol levels,
- calcium channel blockers, such as amlodipine, used to treat high blood pressure,
- theophylline, used to treat asthma,
- warfarin or phenprocoumon, used as anticoagulants,
- phenytoin, used to treat seizures,
- cyclosporin, used in organ transplants as an immunosuppressant,
- benzodiazepines, such as temazepam, used to calm anxiety.
Regarding vaccines, see the previous warnings section.
Because there is no clinical experience, the use of Tyenne with other biologic medicines used to treat RA, sJIA, or pJIA is not recommended.
Pregnancy and breastfeeding
Tyenne must not be used during pregnancy, unless clearly necessary. Talk to your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant.
Women of childbearing age must use effective contraceptive methods during and up to 3 months after finishing treatment.
Stop breastfeeding if you start treatment with Tyenne, and consult your doctor. Before restarting breastfeeding, at least 3 months must have passed since your last treatment with Tyenne. It is not known if Tyenne passes into breast milk.
Data available so far do not suggest that this treatment has any effect on fertility.
Driving and using machines
This medicine may cause dizziness, if you feel dizzy, do not drive or use machines.
Tyenne contains sodium
This medicine contains 0.24 mg of sodium (main component of cooking/table salt) per ml. This is equivalent to 0.012% of the maximum recommended daily intake of sodium for an adult. However, Tyenne is diluted in a solution for infusion of sodium chloride 9 mg/ml (0.9%) or 4.5 mg/ml (0.45%). This should be taken into account in patients on a controlled sodium diet.
3. How to use Tyenne
Follow exactly the administration instructions of this medicine given by your doctor, pharmacist, or nurse. If you are unsure, talk to your doctor, pharmacist, or nurse.
Treatment should be initiated by a healthcare professional experienced in the diagnosis and treatment of RA, sJIA, pJIA, or GCA.
Adults with RA or GCA
The recommended dosefor all adults with RA (rheumatoid arthritis) or GCA (giant cell arteritis) is 162 mg (the content of one pre-filled pen) administered once a week.
Adolescents with sJIA (12 years of age and older)
The usual dose of Tyenne depends on the patient's weight.
- If the patient weighs less than 30 kg: the dose is 162 mg (the content of 1 pre-filled pen), once every 2 weeks.
- If the patient weighs 30 kg or more: the dose is 162 mg (the content of 1 pre-filled pen), once a week.
Adolescents with pJIA (12 years of age and older)
The usual dose of Tyenne depends on the patient's weight.
- If the patient weighs less than 30 kg: the dose is 162 mg (the content of 1 pre-filled pen), once every 3 weeks.
- If the patient weighs 30 kg or more: the dose is 162 mg (the content of 1 pre-filled pen), once every 2 weeks.
Tyenne is administered by injection under the skin (subcutaneously). At the start, your doctor or nurse may inject Tyenne for you. However, your doctor may decide that you can inject Tyenne yourself. In this case, you will receive information on how to self-inject Tyenne. Parents and caregivers will be trained on how to inject Tyenne to patients who cannot inject themselves.
Talk to your doctor if you have any questions about how you or an adolescent you care for can self-administer an injection. At the end of this leaflet, you will find detailed "administration instructions".
If you use more Tyenne than you should
Since Tyenne is administered in a pre-filled pen, it is unlikely that you will be given too much. However, if you are concerned, talk to your doctor, pharmacist, or nurse.
If an adult with RA or GCA or an adolescent with sJIA misses or forgets a dose
It is very important to use Tyenne exactly as your doctor prescribes. Keep a record of your next dose.
- If you miss your weekly dose within 7 days, take your dose on the next scheduled day.
- If you miss your every 2 weeks dose within 7 days, inject a dose as soon as you remember and take your next dose according to your original schedule.
- If you miss your weekly or every 2 weeks dose for more than 7 days or are unsure when to inject Tyenne, call your doctor or pharmacist.
If an adolescent with pJIA misses or forgets a dose
It is very important to use Tyenne exactly as your doctor prescribes. Keep a record of your next dose.
- If you miss a dose within 7 days, inject a dose as soon as you remember and administer the next dose according to your original schedule.
- If you miss a dose for 7 days or more, or are unsure when to inject Tyenne, call your doctor or pharmacist.
If you stop treatment with Tyenne
Do not stop treatment with Tyenne without talking to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Side effects may occur up to at least 3 months after your last dose of Tyenne.
Serious side effects: talk to your doctor immediately.
These are common: May affect up to 1 in 10 people
Allergic reactionsduring or after the infusion:
- difficulty breathing, chest tightness, or dizziness,
- rash, itching, hives, swelling of the lips, tongue, or face.
If you experience any of these symptoms, talk to your doctor immediately.
Signs of serious infections:
- fever and chills,
- mouth or skin blisters,
- stomach pain.
Signs and symptoms of liver toxicity:
May affect up to 1 in 1,000 people
- fatigue,
- abdominal pain,
- jaundice (yellowing of the skin or eyes).
If you notice any of these symptoms, tell your doctor immediately.
Very common side effects:
May affect more than 1 in 10 people
- upper respiratory tract infections, with typical symptoms such as cough, nasal congestion, runny nose, sore throat, and headache,
- high levels of fat in the blood (cholesterol),
- reactions at the injection site.
Common side effects:
May affect up to 1 in 10 people
- lung infection (pneumonia),
- shingles (herpes zoster),
- fever (oral herpes), blisters,
- skin infections (cellulitis), sometimes with fever and chills,
- rash and itching, hives,
- allergic reactions (hypersensitivity),
- eye infection (conjunctivitis),
- headache, dizziness, high blood pressure,
- mouth ulcers, stomach pain,
- fluid retention (edema) in the lower legs, weight gain,
- cough, shortness of breath,
- low white blood cell count in blood tests (neutropenia, leucopenia),
- abnormal liver function tests (elevated transaminases),
- increased bilirubin measured by blood tests,
- low levels of fibrinogen in the blood (protein involved in blood clotting).
Uncommon side effects:
May affect up to 1 in 100 people
- diverticulitis (fever, nausea, diarrhea, constipation, stomach pain),
- swollen and red areas in the mouth,
- high levels of fat in the blood (triglycerides),
- stomach ulcers,
- kidney stones,
- hypothyroidism.
Rare side effects:
May affect up to 1 in 1,000 people
- Stevens-Johnson syndrome (skin rash that can lead to severe skin peeling),
- fatal allergic reactions (anaphylaxis),
- liver inflammation (hepatitis), jaundice.
Very rare side effects:
May affect up to 1 in 10,000 people
- low white blood cell, red blood cell, and platelet count in blood tests,
- liver failure.
Additional side effects in children and adolescents with sJIA or pJIA
Side effects in children and adolescents with sJIA or pJIA are generally similar to those in adults. Some side effects that are more common in children and adolescents are: inflammation of the nose and throat, headache, nausea, and low white blood cell count.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tyenne
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the vials in the outer packaging to protect them from light.
6. Container Contents and Additional Information
Tyenne Composition
- The active ingredient is tocilizumab.
Each pre-filled syringe contains 162 mg of tocilizumab in 0.9 ml.
- The other components are L-arginine, L-histidine, L-lactic acid, sodium chloride, polysorbate 80, hydrochloric acid (E507) and/or sodium hydroxide (E524), water for injectable preparations.
Product Appearance and Container Contents
Tyenne is an injection solution. The solution is clear and colorless to pale yellow.
Tyenne is supplied in 0.9 ml pre-filled syringes containing 162 mg of tocilizumab injection solution.
Each container contains 1 or 4 pre-filled syringes. The multiple container contains 12 (3 containers of 4) pre-filled syringes. Only certain container sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Fresenius Kabi Deutschland GmbH
Else-Kroener-Strasse 1
61352 Bad Homburg v.d.Hoehe
Germany
Manufacturer
Fresenius Kabi Austria GmbH
Hafnerstrasse 36
8055 Graz
Austria
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu/.
- Instructions for Use
Read these instructions for use carefully before using the Tyenne pre-filled syringe.
Read and follow the instructions for use that come with the Tyenne pre-filled syringe before starting to use it and each time you get a replacement. There may be new information. This information does not replace consultation with your doctor about your disease or treatment. If you have any questions about using the Tyenne pre-filled syringe, call your doctor.
Important Information
- Read the patient leaflet that comes with the Tyenne pre-filled syringe for
important information you need to know before using it.
- Before using the Tyenne pre-filled syringe for the first time, make sure your doctor shows you the correct way to use it.
- Do notattempt to disassemble the Tyenne pre-filled syringe at any time.
- Always inject the Tyenne pre-filled syringe in the way your doctor has taught you.
Using the Tyenne Pre-filled Syringe
- The pre-filled syringe is for self-injection or administration with the help of a caregiver.
- The pre-filled syringe is for home use.
- When injecting Tyenne, children may self-inject if both the doctor and the caregiver consider it appropriate.
- Do notreuse the pre-filled syringe. The pre-filled syringe is for a single dose (single use).
- Do notshare your pre-filled syringe with another person. You could infect another person or get an infection from them.
- Do notremove the transparent cap from the pre-filled syringe until you are ready to inject.
- Do notuse the pre-filled syringe if it appears damaged or has been dropped.
Storing the Tyenne Pre-filled Syringe
- Store Tyenne in the refrigerator between 2 °C and 8 °C.
- Keep unused pre-filled syringes in the original container to protect them from light.
- Do not freeze. If Tyenne freezes, discard it in a sharps container.
- Keep Tyenne away from heat or direct sunlight.
- Keep the pre-filled syringe out of the reach and sight of children.
- Tyenne can be stored at room temperature between 20 °C and 25 °C in the carton it comes in for up to 14 days.
- Discard (throw away) Tyenne in a sharps container if it has been out of the refrigerator for more than 14 days. Once stored at room temperature, do not put it back in the refrigerator.
Traveling with Tyenne Pre-filled Syringes
- When traveling by air, always check with your airline and doctor about carrying injectable medications with you. Always carry Tyenne in your carry-on baggage because the baggage area may be very cold and it could freeze.
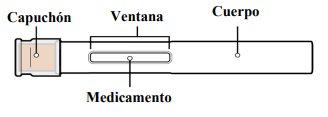
Parts of the Tyenne Pre-filled Syringe
Before Use
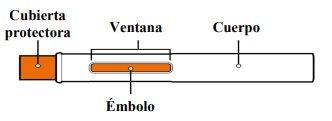
After Use
STEP 1: Prepare Your Injection
1.1. Prepare a flat and clean surface, such as a table or counter, in a well-lit area. | |
1.2. You will also need (not included) (see Figure A):
|
|
1.3. Remove the box containing the pre-filled syringe from the refrigerator. Do notstore the pre-filled syringe outside the refrigerator for more than 14 days without using it. | |
1.4. Check the expiration date on the box to make sure it has not passed (see Figure B). Do notuse the pre-filled syringe if the expiration date has passed. 1.5. If you are opening the box for the first time, check that the box does not show any signs of damage. |
|
Do notuse the pre-filled syringe if the box appears damagedor has been opened. | |
1.6. Open the box and remove a single-use pre-filled syringe. Do nothold the pre-filled syringe by the cap. 1.7. Return any remaining pre-filled syringes to the refrigerator. 1.8. Leave the pre-filled syringe at room temperature on the prepared surface for 45 minutes before using it to allow the medication in the pre-filled syringe to reach room temperature (see Figure C). Note:If you do not do this, the injection may be uncomfortable and may take longer to inject. Do notheat it in any other way, such as in a microwave, hot water, or direct sunlight. |
|
Do notremove the transparent cap from the pre-filled syringe until you are ready to inject to avoid injury. Keep Tyenne out of the reach of children. |
STEP 2: Check the Pre-filled Syringe
2.1. Check that the pre-filled syringe is not cracked or damaged (see Figure D). Do notuse the pre-filled syringe if it shows signs of damage or if it has been dropped. |
|
2.2. Check the label on the pre-filled syringe to make sure that:
Do notuse the pre-filled syringe if the name on the label is not Tyenne or the expiration date has passed. |
|
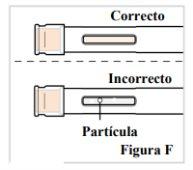
2.3. Look at the medication through the window. Make sure it is clear and colorless to pale yellowand does not contain scales or particles (see Figure F). Note:It is normal to have air bubbles in the medication. Do notinject if the liquid is cloudy, discolored, or has lumps or particles because it may not be safe to use. |
|
STEP 3: Wash Your Hands
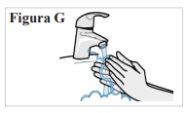
3.1. Wash your hands with water and soap and dry them well with a clean towel (see Figure G). |
|
STEP 4: Choose the Injection Site
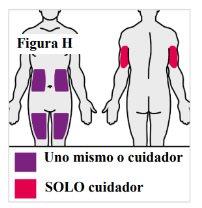
4.1. If you are giving yourself the injection, you can use:
Note: Choose a different place for each injection to reduce redness, irritation, or other skin problems. Do notinject into painful (sensitive), bruised, red, hard, scaly, or damaged skin, or skin with lesions, moles, scars, stretch marks, or tattoos. Do notuse the pre-filled syringe through clothing. |
|
STEP 5: Clean the Injection Site
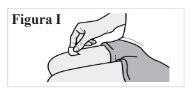
5.1. Clean the skin at the injection site with an alcohol swab (see Figure I). Let the skin dry. Do notblow or touch the area after cleaning. |
|
STEP 6: Give Yourself the Injection
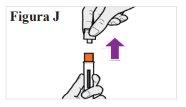
6.1. When you are ready to inject, hold the pre-filled syringe in one hand with the transparent cap facing up. With the other hand, firmly pull the transparent cap straight off without twisting it (see Figure J). Note:Use the pre-filled syringe immediatelyafter removing the cap to avoid contamination. Do notattempt to recap the needle at any time, not even at the end of the injection. Do nottouch the needle cap (the orange part at the tip of the pre-filled syringe) because you could accidentally prick yourself. 6.2. Discard the transparent cap. 6.3. Turn the pre-filled syringe so that the orange needle cover points downwards. |
|
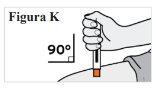
6.4. Place your hand over the pre-filled syringe so that you can see the window. 6.5. Place the pre-filled syringe against your skin at a 90-degree angle (straight) (see Figure K). Note:To make sure you inject under the skin (into the fatty tissue), do not hold the pre-filled syringe at an angle. Note:You do not need to pinch the skin. |
|
To make sure you inject the full dose, read all the steps from 6.6 to 6.9 before starting: |
|
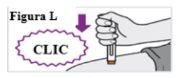
6.6. With a single motion, firmly push the pre-filled syringe against your skin until you hear a first click. The orange plunger will move through the window during the injection (this means the injection has started) (see Figure L). |
|
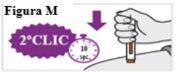
6.7. WAIT and keep the pre-filled syringe in place until you hear a second click. This may take up to 10 seconds. Continue HOLDING(see Figure M). |
|
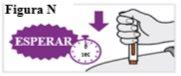
6.8. Wait and count slowly to 5 after hearing the second click. Continue HOLDINGthe pre-filled syringe in place to make sure you inject a full dose (see Figure N). Do notlift the pre-filled syringe until you are sure that 5 seconds have passed and the injection is complete. |
|
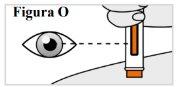
6.9. While holding the pre-filled syringe in place, check the window to make sure the orange plunger has appeared completely in the window and has stopped moving (see Figure O), Note:If the orange plunger has not moved all the way down or if you think you have not received a full injection, call your doctor. Do notattempt to repeat the injection with a new pre-filled syringe. |
|
STEP 7: Remove and Check the Pre-filled Syringe
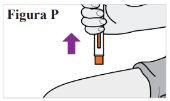
7.1. Once the injection is complete, remove the pre-filled syringe from your skin (see Figure P). Note:The needle cap will slide down and cover the needle. Do notrecap the pre-filled syringe. |
|
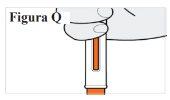
7.2. Check the window to make sure the orange plunger has moved all the way down (see Figure Q). Note:If the orange plunger has not moved all the way down or if you think you have not received a full injection, call your doctor. Do notattempt to repeat the injection with a new pre-filled syringe. |
|
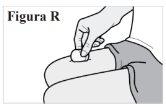
7.3. If you see blood at the injection site, press a cotton ball or gauze against the skin until it stops bleeding (see Figure R). Do notrub the injection site. |
|
STEP 8: Dispose of Your Pre-filled Syringe
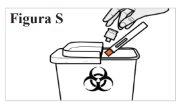
8.1. Place the used pre-filled syringe in a sharps container immediately after use (see Figure S). Do notput the transparent cap back on the pre-filled syringe. Do notthrow (discard) the pre-filled syringe in household trash. Do notreuse the pre-filled syringe. If you do not have a sharps container, you can use a household container that:
When the sharps container is almost full, you will need to follow local guidelines for the proper disposal of the container. Do notthrow (discard) the used sharps container in household trash unless local regulations allow it. Do notrecycle the used sharps container. Always keep the sharps container out of the reach of children. |
|
STEP 9: Record Your Injection
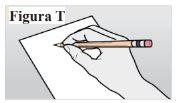
9.1. Record the date and site of the injection (see Figure T). Note:This will help you remember when and where you should give your next injection. |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TYENNE 162 mg INJECTABLE SOLUTION IN PRE-FILLED PENDosage form: INJECTABLE, 162 mgActive substance: tocilizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription requiredDosage form: INJECTABLE, 162 mgActive substance: tocilizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription requiredDosage form: INJECTABLE PERFUSION, 20 mg/mlActive substance: tocilizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription required
Online doctors for TYENNE 162 mg INJECTABLE SOLUTION IN PRE-FILLED PEN
Discuss questions about TYENNE 162 mg INJECTABLE SOLUTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions