
TIORFAN INFANTIL 4 mg/mL ORAL SUSPENSION


How to use TIORFAN INFANTIL 4 mg/mL ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Tiorfan Infant 4mg/ml Oral Suspension is and what it is used for
- What you need to know before your child takes Tiorfan Infant 4mg/ml Oral Suspension
- How to take Tiorfan Infant 4mg/ml Oral Suspension
- Possible Side Effects
- Storage of Tiorfan Infant 4mg/ml Oral Suspension
- Packaging Contents and Additional Information
Introduction
Package Leaflet: Information for the User
Tiorfan Infant 4mg/ml Oral Suspension
racecadotril
Read all of this leaflet carefully before your child starts taking this medicine because it contains important information for them.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for your child only. Do not give it to others, even if they have the same symptoms as your child, as it may harm them.
- If your child experiences side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What Tiorfan Infant 4mg/ml Oral Suspension is and what it is used for
- What you need to know before your child takes Tiorfan Infant 4mg/ml Oral Suspension
- How to take Tiorfan Infant 4mg/ml Oral Suspension
- Possible side effects
- Storage of Tiorfan Infant 4mg/ml Oral Suspension
- Contents of the pack and further information
1. What Tiorfan Infant 4mg/ml Oral Suspension is and what it is used for
Tiorfan Infant 4mg/ml Oral Suspension is a medicine for the treatment of diarrhea.
Tiorfan Infant 4mg/ml Oral Suspension is used in addition to oral rehydration and dietary measures for the treatment of symptoms of acute diarrhea in infants and children over 3 months and weighing 7 kg or more. It should be used together with fluid intake as much as possible and usual dietary measures, when these measures are not sufficient on their own to control diarrhea, and when the cause of diarrhea cannot be treated.
When the cause of diarrhea can be treated, racecadotril may be administered as complementary treatment.
2. What you need to know before your child takes Tiorfan Infant 4mg/ml Oral Suspension
- If your doctor has told you that your child has an intolerance to some sugars, contact your doctor before administering this medicine, as it contains sucrose.
Do not give Tiorfan Infant 4mg/ml Oral Suspension
- If your child is allergic to racecadotril or any of the other ingredients of this medicine (listed in section 6).
- If your child has ever developed a severe skin rash, blistering, or peeling of the skin after taking racecadotril.
Warnings and Precautions
Consult your doctor or pharmacist before giving Tiorfan Infant 4mg/ml to your child if:
- Your child is under 3 months or weighs less than 7 kg.
- There is blood or pus in your child's stool and if your child has a fever. The cause of diarrhea may be a bacterial infection that needs to be treated by your doctor.
- Your child has chronic diarrhea or diarrhea caused by antibiotics.
- Your child has kidney disease or liver failure.
- Your child has more than 6 liquid stools per day or has diarrhea accompanied by weight loss.
- Your child has prolonged or uncontrolled vomiting.
- Your child has diabetes (see section "Tiorfan Infant 4mg/ml Oral Suspension contains sodium, sodium benzoate, sucrose, and propylene glycol").
There have been reports of hypersensitivity/angioedema (swelling) in patients with racecadotril (the active ingredient of this product). Angioedema of the face, extremities, lips, mucous membranes, etc., or swelling of the upper respiratory tract, e.g., tongue, glottis, and/or larynx (throat), may occur. These can occur at any time during therapy. If you experience these side effects, stop treatment immediately and contact your doctor.
Patients with a history of angioedema (swelling) not related to treatment with racecadotril may be at higher risk of angioedema.
The concomitant use of this medicine and others may increase the risk of angioedema (see "Other medicines and Tiorfan Infant 4mg/ml Oral Suspension").
There have been reports of skin reactions with the use of this product. These are usually mild to moderate. When severe skin reactions occur, treatment should be stopped immediately. Do not reintroduce racecadotril.
Be careful with racecadotril:
Severe skin reactions, including drug reaction with eosinophilia and systemic symptoms (DRESS), have been reported with racecadotril treatment. Suspend the use of racecadotril and seek immediate medical attention if you notice any of the symptoms related to these severe skin reactions described in section 4.
This treatment is given in addition to oral rehydration and dietary measures. Your doctor will decide if your child needs an oral rehydration solution. You will need to follow the conditions of use of the oral rehydration solution prescribed by your doctor and follow the dietary advice.
Other medicines and Tiorfan Infant 4mg/ml Oral Suspension
Tell your doctor or pharmacist if your child is taking, has recently taken, or might take any other medicines, including:
- An ACE inhibitor (e.g., perindopril or ramipril) to lower blood pressure and facilitate heart work.
- Angiotensin II antagonists (e.g., candesartan or irbesartan) to treat high blood pressure and heart failure.
If you have given or have recently given another medicine to your child, including non-prescription medicines, talk to your doctor or pharmacist.
Pregnancy, Breastfeeding, and Fertility
Pregnancy
Consult your doctor or pharmacist before using any medicine.
Based on available data, this medicine is not recommended during pregnancy, at any stage.
Breastfeeding
In the absence of information on the transmission of the active ingredient through breast milk, this medicine should not be used during breastfeeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and Using Machines
This medicine has little or no effect on the ability to drive or use machines.
Tiorfan Infant 4mg/ml Oral Suspension contains sodium, sodium benzoate, sucrose, and propylene glycol
If your doctor has told you that your child has an intolerance to some sugars, ask your doctor before giving Tiorfan Infant 4 mg/ml.
This medicine contains 225 mg of sucrose per dose-kg. This should be taken into account for patients with diabetes mellitus.
Patients with fructose intolerance, glucose-galactose malabsorption syndrome, or sucrose-isomaltase deficiency (rare hereditary diseases) should not take this medicine.
This medicine contains 0.84 mg of sodium (the main component of kitchen/table salt) per dose-kg.
The amount of sodium should be included in the maximum recommended nutritional amount by the WHO, corresponding to 1,500 mg for children.
This medicine contains 1.13 mg of benzoate per dose-kg.
Sodium benzoate may increase the risk of jaundice (yellowing of the skin and eyes) in newborns (up to 4 weeks).
This medicine contains 1.06 mg of propylene glycol per dose-kg.
3. How to take Tiorfan Infant 4mg/ml Oral Suspension
Always give this medicine to your child exactly as your doctor has told you. You should consult your doctor or pharmacist if you are not sure.
This medicine is an oral suspension with a characteristic strawberry flavor
Only for infants and children between 7 kg and 52 kg.
The recommended dose is based on the child's body weight. It is 1.5 mg/kg/dose (which corresponds to a dose-kg).
Day one: Give a first dose immediately to your child and, depending on the time of the first dose, give up to a maximum of 2 additional doses distributed throughout the day, without exceeding 3 doses in the day. Doses should be administered preferably at the start of the three main meals.
Following days: Give 3 doses distributed throughout the day, preferably at the start of the three main meals.
The maximum daily total dose is 3 doses.
The medicine is administered orally using a syringe (graduated in kg of body weight) that provides a dose of 1.5 mg of racecadotril per graduation point indicated in kg.
For each dose:
- Infants and children up to 26 kg: use the syringe, filling it up to the graduation point that indicates the weight of the infant or child.
- Children between 27 and 38 kg (see the table below): fill the syringe once up to the 13 kg graduation point and administer the suspension to your child. Fill the syringe a second time up to a total equivalent to the child's weight and administer the suspension again to the child.
- Children between 39 and 52 kg (see the table below): fill the 10 ml syringe once up to the 26 kg graduation point and administer the suspension to your child. Fill the 10 ml syringe a second time up to a total equivalent to the child's weight and administer the suspension again to your child.
- For weights exceeding 52 kg, use more suitable pharmaceutical forms.
Child's weight | Graduation for the first filling of the syringe | Graduation for the second filling of the syringe |
27 kg | 13 kg | 14 kg |
28 kg | 13 kg | 15 kg |
29 kg | 13 kg | 16 kg |
30 kg | 13 kg | 17 kg |
31 kg | 13 kg | 18 kg |
32 kg | 13 kg | 19 kg |
33 kg | 13 kg | 20 kg |
34 kg | 13 kg | 21 kg |
35 kg | 13 kg | 22 kg |
36 kg | 13 kg | 23 kg |
37 kg | 13 kg | 24 kg |
38 kg | 13 kg | 25 kg |
39 kg | 26 kg | 13 kg |
40 kg | 26 kg | 14 kg |
41 kg | 26 kg | 15 kg |
42 kg | 26 kg | 16 kg |
43 kg | 26 kg | 17 kg |
44 kg | 26 kg | 18 kg |
45 kg | 26 kg | 19 kg |
46 kg | 26 kg | 20 kg |
47 kg | 26 kg | 21 kg |
48 kg | 26 kg | 22 kg |
49 kg | 26 kg | 23 kg |
50 kg | 26 kg | 24 kg |
51 kg | 26 kg | 25 kg |
52 kg | 26 kg | 26 kg |
Duration of Treatment
Your doctor will tell you the duration of treatment with Tiorfan Infant 4 mg/mL oral solution. It should be continued until your child has two normal stools, without exceeding 7 days.
Method of Administration
Oral use.
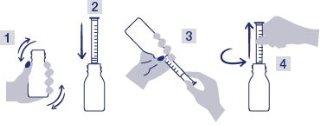
- Shake the bottle vigorously before use. Diagram 1
- Open the bottle by turning and pressing down the child-resistant closure
- Insert the syringe completely into the sampling tip. Diagram 2
- To fill the syringe, hold the bottle upside down. Hold the syringe firmly in place and pull the plunger slowly and continuously to the graduation point in kg. Diagram 3
- Put the bottle back upright and remove the syringe. Diagram 4
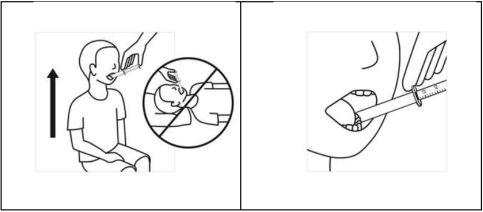
- Keep the child upright during administration. Insert the syringe into the child's mouth without forcing and directing it to the inner surface of the cheek. Administer the suspension completely, while pushing the plunger slowly and gradually.
- After each use, disassemble the oral syringe, rinse it with water, and dry it.
The use of this syringe for oral administration is strictly reserved for the administration of a dose-kg of TIORFAN 4 mg/mL.
To compensate for the loss of fluid due to your child's diarrhea, this medicine should be used together with adequate fluid and electrolyte replacement. The best replacement of fluids and electrolytes is achieved with an oral rehydration solution (consult your doctor or pharmacist if you are not sure).
If you give more Tiorfan Infant 4mg/ml than you should
In case of overdose or accidental ingestion, go immediately to a medical center or consult your doctor, pharmacist, or call the Toxicology Information Service (telephone 91 562 04 20), indicating the medicine and the amount taken.
If you forget to give Tiorfan Infant 4mg/ml
Do not give a double dose to make up for the doses that you have missed giving to your child. Give the next dose.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You must stop giving Tiorfan Infant 4mg/ml to your child and consult a doctor immediately if your child experiences symptoms of angioedema such as:
- Swelling of the face, tongue, or throat
- Difficulty swallowing
- Hives and difficulty breathing
Suspend the use of racecadotril and seek immediate medical attention if you notice any of the following symptoms:
- Widespread skin rash, high body temperature, and enlarged lymph nodes (DRESS syndrome)
- Difficulty breathing, swelling, dizziness, rapid heartbeat, sweating, and feeling of loss of consciousness, symptoms of a severe and sudden allergic reaction.
Uncommon side effects(reported in at least 1 in 1,000 patients but less than 1 in 100 patients):
Tonsillitis (inflammation of the tonsils), rash (skin rashes), and erythema (redness of the skin)
Frequency not known(cannot be estimated from the available data):
Multiple erythema (pink lesions on the extremities and mouth), tongue edema, lip swelling, eyelid edema, facial edema, facial angioedema (subcutaneous inflammation affecting several parts of the body), hives, nodular erythema (inflammation in the form of a lump under the skin), maculopapular rash (rash with small hard and pustular lesions), pruritus (itching affecting the whole body), prurigo (skin lesions that cause itching).
Reporting of side effects
If your child experiences any type of side effect, consult your doctor or pharmacist, even if it is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tiorfan Infant 4mg/ml Oral Suspension
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date that appears on the bottle. The expiry date is the last day of the month indicated.
Do not store above 25°C.
Once opened, do not use the contents of this bottle after 10 days.
Do not use this medicine if you notice visible signs of deterioration.
After completing the treatment, return the box, including the oral syringe and the bottle, to your pharmacist so that they can dispose of it properly and appropriately.
Medicines should not be thrown away via wastewater or household waste. Deposit the packaging and medicines that you no longer need in the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines that you no longer need. This way, you will help protect the environment.
6. Packaging Contents and Additional Information
Composition of Tiorfan infantil 4mg/ml oral suspension
The active ingredient is racecadotril.
Each ml of oral suspension contains 4 mg of racecadotril.
The other components are:
Sodium benzoate, hydroxyethylcellulose, xanthan gum, sucrose, sodium citrate, lactic acid (for pH adjustment), strawberry flavor (contains propylene glycol). See section 2.
Appearance of the product and packaging contents
This medication is an oral suspension with a characteristic strawberry odor.
Packaging:
PET bottle of 50 ml with child-resistant safety cap and 10 ml graduated syringe in kg. Box of 1.
PET bottle of 180 ml with child-resistant safety cap and 10 ml graduated syringe in kg. Box of 1.
Only some pack sizes may be marketed.
Marketing authorization holder
Bioprojet Pharma
9 RUE RAMEAU
75002 Paris
France
Manufacturer
Unither Liquid Manufacturing
1-3 ALLEE DE LA NESTE
31770 COLOMIERS
FRANCE
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
France: Tiorfan 4 mg/mL Nourrissons et Enfants, suspension buvable.
Belgium: Tiorfix nourrissons et enfants 4 mg/ml suspension buvable. Tiorfix zuigelingen en kinderen 4 mg/ml suspensie voor oraal gebruik. Tiorfix Säuglinge und Kinder 4 mg/ml Suspension zum Einnehmen.
Germany: Tiorfan 4 mg/ml Suspension zum Einnehmen.
Ireland: Hidrasec Infants and Children 4mg/mL Oral Suspension.
Italy: Tiorfan.
Luxembourg: Tiorfix 4 mg/ml nourrissons et enfants, suspension buvable.
Spain: Tiorfan infantil 4 mg/ml oral suspension
Date of last revision of this leaflet:
April 2024
Other sources of information
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TIORFAN INFANTIL 4 mg/mL ORAL SUSPENSIONDosage form: CAPSULE, 100 mgActive substance: racecadotrilManufacturer: Bioprojet FerrerPrescription not requiredDosage form: CAPSULE, 100 mgActive substance: racecadotrilManufacturer: Bioprojet FerrerPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 10 mg racecatrodilActive substance: racecadotrilManufacturer: Bioprojet FerrerPrescription required
Online doctors for TIORFAN INFANTIL 4 mg/mL ORAL SUSPENSION
Discuss questions about TIORFAN INFANTIL 4 mg/mL ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















