TESTAVAN 20 mg/g TRANSDERMAL GEL

How to use TESTAVAN 20 mg/g TRANSDERMAL GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Testavan®20 mg/g transdermal gel
Testosterone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Testavan and what is it used for
- What you need to know before you use Testavan
- How to use Testavan
- Possible side effects
- Storing Testavan
- Contents of the pack and other information
1. What is Testavan and what is it used for
What is TestavanTestavan is a clear gel that contains testosterone, a male hormone that is naturally produced in the body.
Testavan is used to replace testosterone in adult men when they do not produce enough natural testosterone - this is called ‘hypogonadism’. This medicine helps to increase the level of testosterone to normal levels.
What does testosterone do
The body naturally produces testosterone in the testicles.
- It helps to produce sperm, develop and maintain male characteristics, such as a deep voice and body hair.
- It is necessary for normal sexual function and sex drive.
- It also helps to maintain muscle size and strength.
What is Testavan used for
Testavan is used as testosterone replacement therapy in men to treat various health problems caused by a lack of testosterone (male hypogonadism). This should be confirmed by carrying out two separate measurements of testosterone in the blood and also by the presence of clinical signs and symptoms such as:
- impotence
- infertility
- low sex drive
- fatigue
- depressive mood
- bone loss due to low hormone levels
- partial loss of secondary sexual characteristics such as changes in voice and fat distribution
- partial loss of facial and body hair.
2. What you need to know before you use Testavan
Who can use Testavan
- Only men can use Testavan.
- Young men under 18 years of age should not use this medicine.
- Women, regardless of their age, should not use this product.
- Do not allow women (especially pregnant or breast-feeding women) or children to come into contact with Testavan gel or with the areas of skin where Testavan has been applied.
Do not use Testavan:
- If you are allergic to testosterone or any of the other ingredients of this medicine (listed in section 6)
- If you have or suspect you have prostate cancer
- If you have or suspect you have breast cancer (a rare condition in men).
Warnings and precautions
Treatment with testosterone may speed up the progress of an existing prostate cancer. Your doctor will carry out the necessary examinations before you can use Testavan and will monitor you by carrying out regular blood tests and prostate examinations.
Consult your doctor before you start using Testavan, if you have or have had any of the following conditions:
- you have difficulty urinating due to an enlarged prostate gland
- you have bone cancer, your doctor should check your calcium levels
- you have high blood pressure or are being treated for high blood pressure, as Testavan may increase blood pressure
- you have a serious heart, liver or kidney disease, treatment with Testavan may cause serious complications in the form of fluid retention, which can be accompanied by congestive heart failure (fluid overload in the heart)
- you have a heart disease called ischaemic heart disease (a disease that affects the blood supply to the heart)
- you have blood clotting problems:
- thrombophilia (a blood clotting abnormality - that increases the risk of thrombosis - blood clots in blood vessels)
- factors that increase the risk of blood clots in a vein: previous blood clots in a vein; smoking; obesity; cancer; immobility; if a close family member has had a blood clot in the leg, lung or other organ at a young age (for example, below 50 years); or as you get older.
- How to recognize a blood clot: painful swelling of a leg or sudden change in skin color, for example paleness, redness or blueness, sudden difficulty breathing, sudden unexplained cough that may bring up blood; or sudden chest pain, severe dizziness or fainting, severe stomach pain, sudden loss of vision. Seek urgent medical attention if you experience any of these symptoms.
- if you suffer from epilepsy
- if you suffer from migraines
- if you have difficulty breathing when you sleep - this is more likely if you are overweight or have a chronic lung disease.
If you have any of these conditions (or are unsure), consult your doctor or pharmacist before using Testavan - use of this medicine may worsen these conditions.
If you develop skin reactions at the application sites of Testavan, seek medical advice. You may need to stop treatment with Testavan.
Your doctor will carry out the following blood tests before and/or during treatment: testosterone levels in the blood, full blood count.
Athletes are informed that this medicine contains testosterone which may result in a positive doping control test.
Testosterone should not be used to increase muscle mass or improve physical strength in healthy individuals.
At the time of, or immediately after, daily application of Testavan, you should not use moisturisers or sunscreens on the area where the gel has been applied.
How to prevent transfer of Testavan to others
Testavan can be transferred to others through direct skin-to-skin contact. This can cause an increase in testosterone levels in the other person's body, which can be dangerous. Transfer can occur through a single contact or gradually through small contacts over time.
- This is particularly important for women and children as they usually have low levels of testosterone in their bodies.
- Pregnant women should not come into contact with Testavan. If your partner is pregnant, be careful and try to protect her from any possible contact with the medicine and the application area.
To avoid transferring the gel from your skin to another person, you should:
- Use the applicator to apply the medicine instead of your fingers.
- Wash your hands with water and soap immediately if your hands come into contact with Testavan.
- Cover the treated area with clothing after the gel has dried.
- Shower or bathe, or put on clothing to cover the application area (for example, a shirt), before having close skin-to-skin contact with someone.
In order to ensure the safety of your partner, wash the treated area with water and soap, for example by showering before sexual intercourse, or if this is not possible, wear a shirt that covers the application area at the time of contact.
What to do if someone else is exposed to Testavan
If someone else touches the gel or comes into direct contact with a skin area treated with the gel, they should wash the skin area with water and soap as soon as possible. The longer the gel stays on the skin before washing, the greater the likelihood that the person will absorb testosterone.
In case of transfer of Testavan to another person, pay attention to any possible changes in that person's body or behaviour. If someone close to you develops any of the following symptoms, they should see a doctor:
- Acne
- Deepening of the voice
- Increased growth of facial or body hair
- Changes in menstrual cycle
- Precocious puberty, increased genital size or changes in sexual behaviour in children
Other medicines and Testavan
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This includes medicines obtained without a prescription and herbal remedies. Testavan may affect the way these medicines work and you may need a dose adjustment.
In particular, tell your doctor or pharmacist if you are taking:
- medicines to thin the blood (anticoagulants) - Testavan may increase the effect of these medicines;
- corticosteroids or any other medicine that may increase the production of these hormones. Corticosteroids and Testavan may cause the body to retain more fluid;
- insulin to control blood sugar levels (in diabetes); you may need to reduce your insulin dose when you receive Testavan.
If any of the above applies to you (or you are unsure), consult your doctor or pharmacist before using Testavan.
Pregnancy, breast-feeding and fertility
Pregnant or breast-feeding women should not use Testavan.
If your partner is or becomes pregnant, you mustfollow the advice above included in the section ‘How to prevent transfer of Testavan to others’.
Pregnant women should avoid anycontact with skin areas treated with Testavan from a man. This medicine may cause harm to the unborn child. In case of contact, the area should be washed as soon as possible with water and soap.
Women who are breast-feeding should avoid anycontact with skin areas treated with Testavan from a man.
Testavan may cause reversible suppression of sperm production.
Testavan contains propylene glycol
This medicine may cause skin irritation because it contains propylene glycol.
Testavan contains ethanol
This medicine contains 538.70 mg of alcohol (ethanol) in each 1.15 g dose, which is equivalent to 468.40 mg/g (46.84% w/w). This may cause a stinging sensation on damaged skin.
3. How to use Testavan
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
How much to use
- The recommended initial dose of Testavan is 23 mg of testosterone (one pump actuation) applied once a day at approximately the same time each day, preferably in the mornings, although in some patients a higher dose may be required.
- The maximum recommended dose is 69 mg of testosterone per day (three pump actuations).
Your doctor will determine the correct dose of Testavan that you need. To do this, they will perform the following determinations regularly:
- analysis of the amount of medication in the blood
- how well the medication works for you
Consult your doctor if you feel that any of the effects of the medication are too strong or too weak.
Using this medication
It is essential that you read and follow these instructions on how to use Testavan correctly.
Apply Testavan to the skin using the applicator. This is called the "transdermal route," where the medication passes through the skin and into the body.
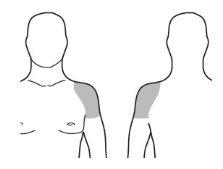
Apply the medication only to healthy, normal, clean, and dry skin on the shoulders or upper arms.
Never apply Testavan to:
- the penis or testicles
- broken or damaged skin
- open sores, wounds, or irritations.
Components you need
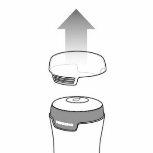
- Testavan gel kit. See Image A.
- Paper towels.
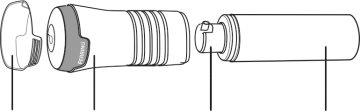
Applicator Cap Pump Actuator
the pump
Image A
Note: Multiple containers have a cap on the top of each pump actuator
Priming the gel pump - only the first time it is used
Before using a new Testavan pump, you must prepare it for use. This is called "priming" the pump:
Remove the applicator from the pump actuator in the case of individual packaging or remove the cap from the pump in the case of multiple packaging.
See Image B.
|
Image B |
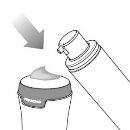
- Prime the pump by pressing the pump actuator downward over a paper towel. Repeat the process until gel appears. See Image C.
|
Image C |
- Do not use the gel obtained during priming, as it may not be the correct dose.
- Discard the used paper towels safely to avoid transferring them to others, including children and pets.
- The Testavan pump is now ready for use.
For daily administration, follow steps 1-4
Step 1: Prepare for Testavan gel application
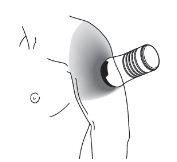
- Select a healthy, normal, clean, and dry skin area on the upper arm and shoulder that will be covered by a shirt sleeve. See Image D.
- Use Testavan gel onlyon the upper arm and shoulder.
Image D |
- Remove the applicator from the pump actuator. See Image E.
|
Image E |
- Remove the cap from the applicator See Image F.
|
Image F |
Step 2: Apply Testavan gel
- Hold the pump actuator with the nozzle over the application surface of the applicator.
- Press the pump actuator downward once. See Image G.
Image G |
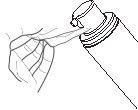
- Use the applicator to spread the gel evenly over the upper arm and shoulder area, making sure not to get the gel on your hands. See Image H.
- Clean up any excess with a paper towel. Discard the used paper towels safely to avoid transferring them to others, including children and pets.
|
Image H |
Check the doseaccording to your doctor's prescription in the table below.
Dose | How to apply it |
23 mg (1 pump actuation) | Apply one pump actuation as shown in Step 2 - do it once. |
46 mg (2 pump actuations) | Apply one pump actuation as shown in Step 2.Repeatto apply another pump actuation on the opposite upper arm and shoulder. |
69 mg (3 pump actuations) | Apply one pump actuation as shown in Step 2.Repeatto apply another pump actuation on the opposite upper arm and shoulder. Repeat againto apply the third pump actuation on the initial upper arm and shoulder where you administered the treatment. |
Step 3: Clean the Testavan applicator
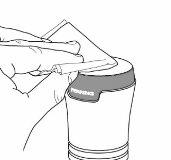
- After use, clean the applicator with a paper towel. See Image I.
|
Image I |
- Discard the used paper towel safely. It is essential to try to avoid transferring it to others, including children and pets.
- REMEMBERto put the cap back on the applicator and replace the applicator on the pump. See Image J.
|
Image J |
- Store the product safely and keep it out of the reach of children.
Step 4: After applying Testavan
- Wash your hands with water and soap immediately if you have gotten gel on them.
- Testavan gel is flammable until it dries. Let the Testavan gel dry before smoking or approaching an open flame.
- Let the application site dry completely before dressing.
- Wear clothing that covers the application area at all times to avoid accidental transfer of the product to others.
- Wait at least 2 hoursbefore showering, swimming, or bathing.
- Wash the application area with water and soap before any situation where skin-to-skin contact with another person may occur.
If you use more Testavan than you should
If you have applied too much medication by mistake, wash the application area with water and soap as soon as possible.
Only use the amount of medication prescribed by your doctor. If you feel irritable, nervous, gain weight, or experience frequent or prolonged erections, these may be symptoms that you are using too much medication.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Testavan
Do not apply a double dose to make up for forgotten doses.
- If the next dose is due in less than 12 hours, do not apply the forgotten dose. Then, apply the next dose as usual.
- If more than 12 hours will pass before the next dose, apply the forgotten dose. Continue with the normal schedule the next day.
If you interrupt treatment with Testavan
Consult your doctor before interrupting treatment with this medication.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Common (may affect up to 1 in 10 people)
- Skin irritation at the application site of the gel (including rash, dryness, and redness).
- High levels of fats (triglycerides) in the blood.
- Hypertension.
- Increased prostate-specific antigen (PSA). PSA is a protein produced by the prostate, which can be used to detect prostate disease.
- Increased hematocrit (percentage of red blood cells in the blood), which is identified through periodic blood tests.
Uncommon (may affect up to 1 in 100 people)
- Increased hemoglobin (the component of red blood cells that carries oxygen) identified through periodic blood tests.
- Headache
Other known adverse reactions associated with testosterone treatment are:
acne, seborrhea, baldness, sweating, increased body hair growth, numbness or tingling of the skin, headache, dizziness, nausea, hot flashes, fluid retention (such as swelling of the ankles), weight gain, sleep apnea, dyspnea, hypersensitivity reactions, feeling unwell, mood changes (aggression, hostility, nervousness, anxiety, depression), insomnia, muscle pain or cramps, breast development, increased red blood cell count, decreased red blood cell count, blood clots, jaundice (liver problems that can sometimes be associated with yellowing of the skin and the whites of the eyes), abnormal liver function tests.
Changes in sexual desire, increased erections, priapism (prolonged and painful erections), testicular disorder, reduced sperm count, difficulty urinating, changes in the prostate, prostate cancer (there is no convincing evidence that testosterone replacement therapy in men with hypogonadism leads to the development of prostate cancer; however, testosterone therapy should be avoided in men known or suspected to have prostate cancer).
Prolonged administration of testosterone may cause changes in electrolyte and water levels in the body.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency's (AEMPS) online system: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Testavan
Keep this medication out of sight and reach of children.
This medication does not require special storage conditions.
Do not use this medication after the expiration date shown on the pump actuator label and the outer packaging after EXP. The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and any unused medications in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medications. This will help protect the environment.
6. Package contents and additional information
Composition of Testavan
The active ingredient is testosterone.
The other excipients are: ethanol 96%, propylene glycol (E 1520), monoethyl ether of diethylene glycol, carbomer 980, tromethamine, disodium edetate, purified water.
One gram of gel contains 20 mg of testosterone. Each pump actuation delivers 1.15 g of gel (1.25 ml), which contains 23 mg of testosterone.
Appearance of the product and package contents
Testavan is a homogeneous, translucent to slightly opalescent gel.
The following package sizes are available with or without an applicator and hygienic cap:
Individual package containing 1 pump actuator
Multiple packages containing 3 pump actuators
Each pump actuator contains 85.5 g of Testavan gel and can dispense 56 measured doses.
Only some package sizes may be marketed
Marketing authorization holder and manufacturer
Marketing authorization holder:
The Simple Pharma Company Limited Ground Floor, 71 Lower Baggot St Dublin D02 P593 Ireland
Manufacturer:
Copea Pharma Europe Limited, Medici House, Unit 2, Ashbourne Manufacturing Park, Ashbourne, Co. Meath, A84 KH58, Ireland
Applicator CAP:
The Simple Pharma Company Limited Ground Floor, 71 Lower Baggot St. Dublin. D02 P593 Ireland
This medication is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
AustriaTestavan 20 mg/g transdermal gel; BelgiumTestarzon 20 mg/g transdermal gel; BulgariaTestavan 20 mg/g ????????? ???; CroatiaTestarzon 20 mg/g transdermal gel; Czech RepublicTestavan; DenmarkTestavan 20 mg/g transdermal gel; EstoniaTestavan; FinlandTestavan 20 mg/g transdermal gel; FranceTestavan 20 mg/g transdermal gel; GermanyTestavan 20 mg/g transdermal gel; GreeceTestarzon 20 mg/g διαδερμικ? γ?λη; HungaryTestarzon 20 mg/g transdermal gel; IrelandTestarzon 20 mg/g transdermal gel; IcelandTestavan 20 mg/g hlaup til notkunar umhúð; ItalyTestavan 20 mg/g transdermal gel; LatviaTestarzon 20 mg/g transdermalais gels; LiechtensteinTestavan 20 mg/g transdermal gel; LithuaniaTestavan 20 mg/g transderminis gelis; LuxembourgTestarzon 20 mg/g transdermal gel; NetherlandsTestavan 20 mg/g gel for transdermal use; NorwayTestavan 20 mg/g transdermal gel; PolandTestavan; PortugalTestavan 20 mg/g transdermal gel; RomaniaTestavan 20 mg/g gel transdermic; SlovakiaTestavan 20 mg/g transdermal gel; SloveniaTestarzon 23 mg/pritisk transdermalni gel; SpainTestavan 20 mg/g transdermal gel; SwedenTestavan 20 mg/g transdermal gel; United KingdomTestavan 20 mg/g transdermal gel.
Date of the last revision of this leaflet: August 2023.
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price45.44 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TESTAVAN 20 mg/g TRANSDERMAL GELDosage form: GEL, 2% testosteroneActive substance: testosteroneManufacturer: Advanz Pharma LimitedPrescription requiredDosage form: INJECTABLE, 1000 mg/dose (250 mg/ml)Active substance: testosteroneManufacturer: Grünenthal Pharma S.A.Prescription requiredDosage form: INJECTABLE, Testosterone Propionate 25 mg/mlActive substance: testosteroneManufacturer: Desma Laboratorio Farmaceutico S.L.Prescription required
Online doctors for TESTAVAN 20 mg/g TRANSDERMAL GEL
Discuss questions about TESTAVAN 20 mg/g TRANSDERMAL GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions