
TAU-KIT SOLUBLE TABLETS


How to use TAU-KIT SOLUBLE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
TAU-KIT®100 mg Soluble Tablets
13C-Urea
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is TAU-KIT® and what is it used for
- What you need to know before taking TAU-KIT®
- How to take TAU-KIT®
- Possible side effects
- Storage of TAU-KIT®
- Package Contents and Additional Information
1. What is TAU-KIT® and what is it used for
TAU-KIT®belongs to the group of medications called other diagnostic agents. TAU-KIT®is a breath test indicated to determine the presence in your stomach of a bacterium called Helicobacter pylori, which may be responsible for the discomfort you are experiencing.
This medication is for diagnostic use only.
2. What you need to know before taking TAU-KIT®
Do not take TAU-KIT®
If you are allergic to the active ingredient or any of the other components of this medication (listed in section 6).
If you have a genetic disorder called Phenylketonuria.
Warnings and Precautions
Consult your doctor before starting to take TAU-KIT®
- A positive test result alone does not constitute an absolute indication for eradication treatment.
Diagnosis with other methods (endoscopy) may be indicated to examine the presence of any other complications, such as ulcers, autoimmune gastritis, or malignant tumors.
- If you have had a partial gastrectomy or are under 5 years old, you should inform your doctor, as there is not enough data to recommend its use in these cases.
- If you have a type of gastritis called atrophic gastritis, the breath test may have false-positive results, so it may be necessary to undergo other studies to confirm the infection.
- If you vomit during the test, it is necessary to repeat it, at least the next day on an empty stomach.
Other Medications and TAU-KIT®
If you are being treated with antibiotics for Helicobacter pyloriinfection, you should wait 4 weeks without medication before performing the diagnostic test with TAU-KIT®, as it could result in a false-negative result.
If you are undergoing treatment with an antacid (medication to relieve heartburn, such as omeprazole, etc.) or an anti-ulcer medication (bismuth salts, etc.), you should wait 2 weeks without medication before performing the diagnostic test.
Tell your doctor or pharmacist if you are using, have recently used, or may need to use other medications.
Using TAU-KIT®with Food and Drinks
As your doctor will indicate, before performing the test, you should fast for at least 6 hours, preferably from the night before, and take 200 ml of a drink rich in citric acid (Citral pylori®) when starting the test, in the case of adults, and 100 ml in the case of children over 5 years old.
Pregnancy, Breastfeeding, and Fertility
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Consult your doctor or pharmacist before using this medication.
Driving and Using Machines
No effects on the ability to drive or use machines have been described at the recommended dose.
Important Information about Some Components of Citral pylori®.
Performing the breath test with 13C-urea requires prior administration of Citric Acid (European Protocol). The TAU-KIT package includes a sachet of Citral pylori® (4.2 g of anhydrous Citric Acid). Each sachet of Citral Pylori® also contains aspartame (E-951) and orange yellow S (E-110)
Each sachet contains 120 mg of aspartame, equivalent to 60 mg/100 ml.
Aspartame contains a source of phenylalanine, which may be harmful in cases of phenylketonuria (PKU), a rare genetic disease in which phenylalanine accumulates because the body is unable to eliminate it properly.
This medication may cause allergic reactions because it contains orange yellow S (E-110). It can cause asthma, especially in patients allergic to acetylsalicylic acid.
3. How to Take TAU-KIT®
Follow your doctor's instructions for taking this medication exactly. If in doubt, consult your doctor or pharmacist again.
TAU-KIT®is a 13C urea breath test used for diagnostic testing, which must be performed in the presence of qualified personnel who will indicate the instructions to follow at all times.
To perform the test correctly, which takes approximately 40 minutes and should be performed preferably in the morning, you must fast for 6 hours, not smoke, and be at rest. The test begins with the ingestion of the contents of the Citral pylori® sachet.
The normal dose is:
Adults over 18 years: one tablet (100 mg of 13C-urea) dissolved in 125 ml of water.
Use in children over 5 years and adolescents:
There is not enough data to recommend its use in patients under 5 years old.
Children over 5 years: half a tablet (50 mg of 13C-urea) dissolved in 50 ml of water.
Method of administration:
The medication is administered orally.
If you vomit while performing the test, a second test should be performed, but not before 24 hours.
Follow these instructions unless your doctor has given you different ones.
If you take more TAU-KIT®than you should
Since the package contains a single tablet of 100 mg of 13C-urea, overdose is not expected. However, if you have taken more TAU-KIT® than you should, consult your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or go to a medical center immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take TAU-KIT®
Since the product consists of a single tablet and is used at a specific time established by your doctor, it is not expected that you will forget to take the medication.
Do not take a double dose to make up for forgotten doses.
If you stop treatment with TAU-KIT®
It is considered that the treatment consists of taking the single tablet with 100 mg of 13C-urea from the package, so it is not expected that there will be an interruption of treatment.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, TAU-KIT® can cause side effects, although not everyone gets them.
No side effects have been reported.
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of TAU-KIT®
Keep this medication out of the sight and reach of children.
No special storage conditions are required.
Do not use this medication if you notice any signs of deterioration in the package.
Expiration Date
Do not use this medication after the expiration date shown on the package, after the abbreviation CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away in drains or trash. Deposit the packages and medications you no longer need in the SIGRE point of your pharmacy. If in doubt, ask your doctor or pharmacist how to dispose of packages and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of TAU-KIT®
- 1 soluble tablet: the active ingredient is: 13C Urea 100 mg
The other components of the tablet are: microcrystalline cellulose, povidone K90, colloidal silica, magnesium stearate, and sodium croscarmellose.
- 1 sachet of Citral pylori: anhydrous Citric Acid, Aspartame (E-951), lemon powder flavor, and orange yellow S (E-110) colorant.
Appearance of the Product and Package Contents
TAU-KIT®is presented in packages with a single soluble tablet containing 100 mg of 13C urea and a sachet of Citral pylori®. It also includes 2 pre-dose sample collection tubes (BASAL), 2 post-dose sample collection tubes (POST), and two flexible tubes, adhesive labels identifying the sample, one of which is placed in the indicated place on the case.
The 50-kit format is presented as 50 kits of 4 air sample collection tubes in each kit, 1 tablet of 13C urea in each kit, and 1 sachet of Citral pylori® in each kit.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder and Manufacturer:
ISOMED PHARMA, S.L.
c/París, nº4, Parque Empresarial Európolis
28232, Las Rozas Madrid
Spain
This package leaflet was last revised inOctober 2019
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
This information is intended only for healthcare professionals.
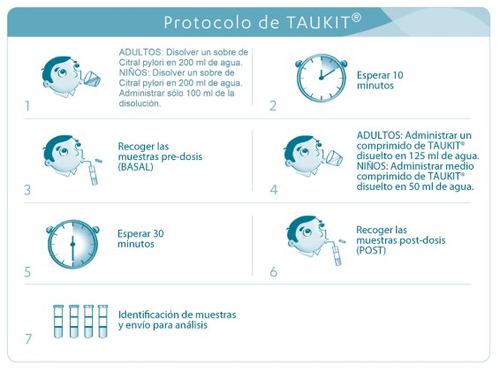
It is essential to follow these instructions precisely to ensure the reliability of the diagnosis.
Specific Instructions:
- The test must be performed in the presence of a qualified person.
- It is recommended to perform the test while at rest.
- The test begins with the ingestion of 1 sachet of Citral pylori® dissolved in 200 ml of water in the case of adults and administration of only 100 ml of the solution in the case of children over 5 years old. The time of ingestion should be noted.
- Ten minutes later, the collection of samples for the determination of the basal value is carried out:
- Take the flexible tube and the two pre-dose sample collection tubes provided with a label where the word “BASAL” appears.
- Remove the cap from one of the sample tubes and unwrap the flexible tube, without bending it, and insert it into the sample collection tube.
- Hold your breath for a few seconds (approx. 30 seconds) so that the concentration of 13CO2 in the exhaled breath is maximum, then exhale gently through the flexible tube until the inner surface of the sample collection tube is covered with condensed vapor.
- Continue exhaling while removing the plastic tube and immediately closing the sample tube with its cap. To close the tube, proceed as follows:
screw until the cap does not turn easily and then turn carefully another quarter turn. If the container remains open for more than 30 seconds, the test could give a false result.
- Fill the second sample tube, provided with a label with the word “BASAL”, with exhalation and following the same procedure described in point 4.
- Prepare the test solution: dissolve the tablet in 125 ml of water in the case of adults and half a tablet in 50 ml of water in the case of children over 5 years old.
- This test solution should be drunk immediately by the patient and the time of ingestion should be noted.
- Thirty minutes after administration of the test solution, samples are collected again in the sample collection tubes provided with a label with the word “POST” and proceed as described in points 4 and 5.
- The sample collection tubes should be sent in the original box for analysis to a qualified laboratory. The sample identification label should be placed in the indicated place on the box.
- Country of registration
- Average pharmacy price30.46 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TAU-KIT SOLUBLE TABLETSDosage form: TABLET, 50 mgActive substance: 13C-ureaManufacturer: Laboratoires Mayoly SpindlerPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, UnknownActive substance: 13C-ureaManufacturer: Infai GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 45 mgActive substance: 13C-ureaManufacturer: Infai GmbhPrescription required
Online doctors for TAU-KIT SOLUBLE TABLETS
Discuss questions about TAU-KIT SOLUBLE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















