
ESPIKUR 50 mg TABLETS


How to use ESPIKUR 50 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Espikur50mg tablets
13C-Urea
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Espikur is and what it is used for
- What you need to know before you take Espikur
- How to take Espikur
- Possible side effects
- Storage of Espikur
- Contents of the pack and other information
1. What Espikur is and what it is used for
This medicinal product is for diagnostic use only.
Espikur is a breath test. After taking the tablet, the breath test will show if your stomach is infected with the bacteria Helicobacter pylori.
Why do you need to take the Espikur test? You may have a gastric infection caused by a bacterium called Helicobacter pylori. Your doctor has prescribed the test for one of the following reasons:
- Your doctor wants to confirm if you have a Helicobacter pyloriinfection.
- You have already been found to be infected with Helicobacter pyloriand have been taking medication to cure the infection. Your doctor will now check if the treatment has been successful.
2. What you need to know before you take Espikur
Do not takeEspikur:
- if you are allergic to 13C-urea or any of the other ingredients of this medicinal product (listed in section 6).
- if you have or suspect you have a stomach infection, as this may cause false results.
- if you have inflammation of the stomach lining (atrophic gastritis), as this may cause false results.
Warnings and precautions
Talk to your doctor or pharmacist before taking Espikur if you have had all or part of your stomach removed.
TakingEspikur with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The test must not be performed within at least:
- 4 weeks after taking medicines for a bacterial infection
- 2 weeks after the last administration of a medicine to reduce stomach acid release.
Both groups of medicines may affect the results of the test. This is especially important after treatment for Helicobacter pyloriinfection.
Pregnancy and breastfeeding
It is not expected that taking the breath test during pregnancy and breastfeeding will have a harmful effect. Espikur can be used during pregnancy and breastfeeding.
Driving and using machines
Espikur has no known effects on the ability to drive or use machines.
3. How to take Espikur
Follow exactly the administration instructions of this medicinal product given by your doctor. If in doubt, consult your doctor or pharmacist again.
You must be fasting for at least 6 hours before performing the breath test. You can drink some water until the test is performed.
Some medicines may affect the result of the test. See the section “Taking Espikur with other medicines”.
The procedure takes approximately 10 minutes.
The recommended dose is one tablet as a single dose. The tablet should be swallowed whole with a glass of water. If the tablet is chewed, the test must be performed again, as this increases the risk of obtaining a false result.
A new test can be performed as soon as the next day.
This medicinal product must not be administered to children under 18 years of age due to lack of data.
In case of doubt, ask your doctor or pharmacist.
If you take more Espikurthan you should
Since only one tablet is provided, overdose is not expected. If in doubt, contact your doctor.
4. Possible side effects
Like all medicines, this medicinal product can cause side effects, although not everybody gets them.
Very rare(may affect up to 1 in 10,000 people)
- stomach pain
- fatigue
- altered sense of smell (parosmia)
Tell your doctor or pharmacist if any of the side effects gets serious.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Agency's website for adverse reaction reporting: www.notificaRAM.es
By reporting side effects, you can help provide more information on the safety of this medicinal product.
5. Storage of Espikur
Keep this medicinal product out of the sight and reach of children.
Do not use this medicinal product after the expiry date which is stated on the label and on the carton after “EXP”. The expiry date is the last day of the month shown.
Store in the original package to protect from light.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
Composition of Espikur
- The active substance is 13C-urea. Each tablet contains 50 mg of 13C-urea.
- The other ingredients are: anhydrous citric acid, anhydrous colloidal silica, sodium croscarmellose, microcrystalline cellulose, magnesium stearate and talc.
Appearance and packaging
Espikur is a white, round, convex tablet, 12 mm in diameter.
Pack sizes:
1 tablet (kit) in aluminium blister pack (with sample tubes and disposable straw).
10 x 1 tablets in aluminium blister pack (without sample tubes and disposable straw).
Breath bags are supplied separately.
Component | 1 tablet (kit) | 10 x 1 tablet |
Tablet in aluminium blister pack | 1 | 10 |
Sample tubes for 00-minute breath sample (blue cap) | 2 | |
Sample tubes for 10-minute breath sample (red cap) | 2 | |
Disposable straw | 1 | |
Leaflet | 1 | 1 |
Bar code labels for sample tubes | 4 | - |
Additional bar code labels | 2 |
Marketing authorisation holder and manufacturer
Marketing authorisation holder:
Laboratoires MAYOLY SPINDLER
6 avenue de l’Europe
78400 Chatou
France
Manufacturer:
Laboratoires MAYOLY SPINDLER
6 avenue de l’Europe
78400 Chatou
France
This medicinal product is authorised in the Member States of theEconomic European Areaunder the following names:
Belgium, Luxembourg, Portugal, Spain, Sweden, Netherlands: Espikur 50 mg
Italy: Helidiag 50 mg
Date of last revision of this leaflet: April 2019
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
It is recommended to perform the test with the patient in a resting position.
If the test is to be performed in the morning, the patient should fast overnight and not have breakfast. If the test is to be performed later in the day, or if fasting may cause problems for the patient, then only a light breakfast is allowed, e.g. tea and toast. Additionally, if the patient has had a heavy meal, they will need to fast for six hours before the test.
Breath samples can be collected using either tubes or breath bags.
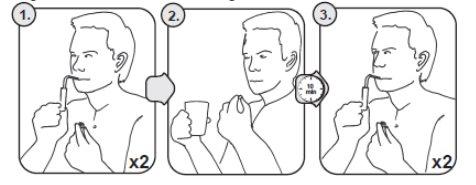
Procedure for the test using sample tubes (see Figure1).
To perform the test, 4 sample tubes with caps and 1 straw are used.
Keep one of the additional bar code labels as a reference label for the patient's medical history.
- The patient must start the procedure with the two 00-MINUTE sample tubes with blue caps.
- Remove the cap.
- Place the straw in the bottom of the sample tube.
- Take a deep breath and exhale slowly into the tube.
- Remove the straw from the tube and close the tube with the cap immediately.
- Check that the cap is properly closed.
- Repeat the test with the second 00-MINUTE sample tube.
- Swallow the tablet with a glass of water. Wait 10 minutes in an upright position (standing or sitting).
- Exhale into the two 10-MINUTE sample tubes with red caps in the same way as described above.
After collecting the samples, the 4 tubes must be labelled according to the image below, placing a bar code label along each tube.
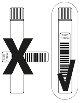
Send the 4 labelled sample tubes for analysis to the local diagnostic laboratory.
Handle the tubes with the samples carefully and avoid any damage that may cause leakage.
Figure 1. How to perform the test using sample tubes.
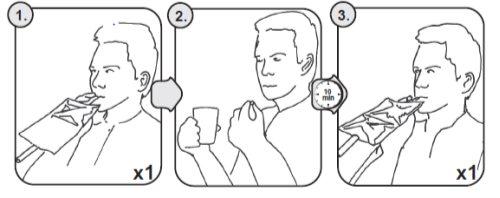
Procedure for the test using breath bags (see Figure2).
To perform the test, 2 individual breath bags or 1 double breath bag and 1 mouthpiece are used.
- Remove the screw cap from the flexible tube of the breath bag and connect the mouthpiece to the flexible tube. Exhale through the mouthpiece to obtain the initial sample (00-MINUTE) of the breath bag. Remove the mouthpiece from the breath bag and close the breath bag with the screw cap.
- Swallow the tablet with a glass of water. Wait 10 minutes in an upright position (standing or sitting).
- Exhale into the unused side of the double breath bag or into another individual breath bag to obtain the test sample (10-MINUTES) of breath bag in the same way as described above.
Mark the breath bags to identify the different samples (e.g. “zero test” and “10-minute test”).
Handle the breath bags with the samples carefully and avoid any damage that may cause leakage.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ESPIKUR 50 mg TABLETSDosage form: ORAL SOLUTION/SUSPENSION, UnknownActive substance: 13C-ureaManufacturer: Infai GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 45 mgActive substance: 13C-ureaManufacturer: Infai GmbhPrescription requiredDosage form: TABLET, 100 mgActive substance: 13C-ureaManufacturer: Isomed Pharma S.L.Prescription required
Online doctors for ESPIKUR 50 mg TABLETS
Discuss questions about ESPIKUR 50 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















