
SOGROYA 10 mg/1.5 ml injectable solution in a prefilled pen


How to use SOGROYA 10 mg/1.5 ml injectable solution in a prefilled pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Sogroya 10mg/1.5ml solution for injection in pre-filled pen
somapacitan
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may have. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Sogroya and what is it used for
- What you need to know before you use Sogroya
- How to use Sogroya
- Possible side effects
- Storage of Sogroya
- Contents of the pack and other information
1. What is Sogroya and what is it used for
Sogroya contains the active substance somapacitan: a long-acting version of the natural growth hormone produced by the body with an amino acid substitution. Growth hormone regulates fat, muscle, and bone composition in adults.
The active substance in Sogroya is produced by "recombinant DNA technology" from cells that have received a gene (DNA) that makes them produce growth hormone. In Sogroya, a small side chain has been added to the growth hormone that binds Sogroya to the protein (albumin) naturally present in the blood to slow down its elimination from the body, allowing the medicine to be administered less frequently.
Sogroya is used to treat growth retardation in children from 3 years of age and adolescents if they have a null or very low production of growth hormone (growth hormone deficiency) and adults with growth hormone deficiency.
Your doctor will assess, based on your response to Sogroya, whether you should continue treatment with Sogroya one year after starting this medicine.
2. What you need to know before you use Sogroya
Do not use Sogroya
- if you or a minor in your care is allergic to somapacitan or any of the other ingredients of this medicine (listed in section 6).
- if you or a minor in your care has a benign or malignant tumor in development. You must have finished anti-tumor treatment before starting treatment with Sogroya. If the tumor grows, you must stop receiving Sogroya.
- if you or a minor in your care has recently undergone open-heart or abdominal surgery or has suffered multiple accidental trauma or has severe respiratory problems or similar conditions.
- in children and adolescents who have stopped growing due to the closure of their growth plates (closed epiphyses), which means that your doctor has indicated to you or the minor in your care that their bones have stopped growing.
In case of doubt, consult your doctor, pharmacist, or nurse.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before using Sogroya if:
- you or a minor in your care has ever had any type of tumor
- you or a minor in your care has a high blood sugar level (hyperglycemia), as you may need to regularly check your blood sugar level and adjust the dose of your diabetes medicine
- you or a minor in your care is receiving corticosteroid replacement therapy because your body does not produce enough (adrenal insufficiency). Talk to your doctor, as you may need regular adjustments
- you or a minor in your care experiences severe headache, vision problems, nausea, or vomiting, as these can be symptoms of increased intracranial pressure (benign intracranial hypertension) and you may need to interrupt treatment
- you or a minor in your care has thyroid problems, you should periodically check your thyroid hormones and may need to adjust the dose of thyroid hormones
- you are a woman taking oral contraceptives or hormone replacement therapy with estrogens, you may need a higher dose of Sogroya. If you stop taking oral estrogens, you may need a lower dose of somapacitan. Your doctor may recommend that you change the way you take estrogens (e.g., transdermal, vaginal) or use another contraceptive method
- you or a minor in your care is seriously ill (e.g., complications from open-heart surgery, abdominal surgery, accidental trauma, acute respiratory failure, or similar diseases). If you have undergone or are going to undergo major surgery, or are going to be hospitalized for any of the above reasons, inform your doctor and remind other doctors who may treat you that you are being treated with growth hormone
- you or a minor in your care suffers from severe stomach pain during treatment with Sogroya, as this could be a symptom of pancreatitis observed in treatments with other growth hormone medicines
- you or a minor in your care suffers from persistent hip or knee pain when walking, or if you or a minor in your care starts to limp during growth hormone treatment. These could be symptoms of a disease that affects the femur where it inserts into the hip (slipped capital femoral epiphysis) and occurs more frequently in children with rapid growth and children with endocrine disorders, including growth hormone deficiency. Inform your doctor if you suffer from persistent pain in any joint.
Changes in the skin at the injection site
The injection site of Sogroya should be rotated to prevent changes in the fatty tissue under the skin, such as thickening of the skin, shrinkage of the skin, or lumps under the skin. Change the injection site on your body every week.
Antibodies
It is not expected that you will develop antibodies against somapacitan. However, in very rare cases, the child may develop antibodies. If your treatment with Sogroya does not work, your doctor will need to check if you have developed antibodies to somapacitan.
Other medicines and Sogroya
Tell your doctor or pharmacist if you or a minor in your care is using, has recently used, or might use any other medicines.
In particular, tell your doctor if you or a minor in your care is taking or has recently taken any of the following medicines.
The reason is that your doctor may need to adjust the doses of your medicines:
- Corticosteroids such as hydrocortisone, dexamethasone, and prednisolone
- Estrogens such as part of oral contraception or hormone replacement therapy with estrogens
- Male sex hormones (androgens) such as testosterone
- Gonadotropins (gonad-stimulating hormones, such as luteinizing hormone and follicle-stimulating hormone), which stimulate the production of sex hormones
- Insulin or other diabetes medicines
- Thyroid hormone medicines such as levothyroxine
- Medicines for epilepsy or seizures (epileptic seizures) such as carbamazepine
- Cyclosporins (immunosuppressant), a medicine that suppresses the immune system response.
Pregnancy
- If you can become pregnant, you must not use Sogroya unless you are using a reliable method of contraception. This is because it is not known whether it could harm the fetus. If you become pregnant during treatment with Sogroya, inform your doctor immediately. If you wish to become pregnant, talk to your doctor, as you may need to stop using the medicine.
Breast-feeding
- It is not known whether Sogroya is excreted in breast milk. Inform your doctor if you are breast-feeding or plan to breast-feed. Your doctor will help you decide whether to stop breast-feeding or stop using Sogroya, considering the benefit of breast-feeding for the baby and the benefit of Sogroya for the mother.
Driving and using machines
Sogroya does not affect the ability to drive and use machines.
Sodium content
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially "sodium-free".
3. How to use Sogroya
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Sogroya is injected under the skin (subcutaneous injection) with a pre-filled pen. You can administer the injection yourself. Your doctor or nurse will show you how to inject Sogroya when you or a minor in your care starts treatment.
When to use Sogroya
- You or a minor in your care should use Sogroya once a week, on the same day of the week if possible.
- The injection can be administered at any time of day.
If you or a minor in your care changes from another weekly growth hormone treatment to Sogroya, it is recommended to continue injecting on the same day of the week.
If you or a minor in your care changes from a daily growth hormone treatment to Sogroya, choose the preferred day for weekly administration and inject the last dose of the daily treatment the day before (or at least 8 hours before) the first dose of Sogroya.
Any change from another type or brand of growth hormone should be made by your doctor.
If it is not possible for you or a minor in your care to inject Sogroya on the usual day of the week, you can administer Sogroya up to 2 days before or 3 days after the scheduled day. The next week, you can inject the next dose as usual.
If necessary, you can change the day of the weekly Sogroya injection, as long as at least 4 days have passed since the last injection. Once the new administration day is selected, you must continue injecting the dose on the same day every week.
How long will you need treatment
You may need Sogroya as long as your body does not produce enough growth hormone.
- If you or a minor in your care is using Sogroya for growth retardation, you will continue using Sogroya until you stop growing.
- If you or a minor in your care still has a growth hormone deficiency after stopping growth, you may need to continue treatment with Sogroya until adulthood.
Do not stop your treatment with Sogroya without talking to your doctor first.
How much to use
Children and adolescents
The dose for children and adolescents depends on body weight.
The recommended dose of Sogroya is 0.16 mg per kg of body weight administered once a week.
Adults
The usual initial dose is 1.5 mg once a week if it is the first time you receive growth hormone treatment. The usual initial dose is 2 mg once a week if you have previously received daily growth hormone treatment (somatropin).
If you are a woman taking oral estrogens (contraceptives or hormone replacement therapy), you may need a higher dose of somapacitan. If you are over 60 years old, you may need a lower dose. See Table 1 below.
Your doctor may increase or decrease the dose gradually and regularly until the right dose is found according to your individual needs and the side effects experienced.
- Do not use more than 8 mg once a week.
- Do not change the dose unless your doctor tells you to.
Table 1 Recommended dose
Adult population with growth hormone deficiency | Recommended initial dose |
Has not previously received daily growth hormone treatment | |
Is ≥18 and <60 years old< p> Is a woman on oral estrogen treatment regardless of age Is 60 years old or older | 1.5 mg/week 2 mg/week 1 mg/week |
Has previously received daily growth hormone treatment | |
Is ≥18 and <60 years old< p> Is a woman on oral estrogen treatment regardless of age Is 60 years old or older | 2 mg/week 4 mg/week 1.5 mg/week |
After reaching the correct dose, your doctor will assess your treatment every 6 to 12 months. You may need to review your body mass index and have blood samples taken.
How to use Sogroya
Your doctor or nurse will show you how to inject Sogroya under the skin.
The best places to inject are:
- the front of the thigh
- the front of the waist (abdomen)
- the buttocks
- the upper arms.
Change the injection site on your body every week.
Detailed instructions on how to inject Sogroya, instructions for use, are included at the end of this leaflet.
If you use more Sogroya than you should
If you or a minor in your care accidentally uses more Sogroya than you should, consult your doctor, as you may need to check your blood sugar levels.
If you forget to use Sogroya
If you or a minor in your care forgets to inject a dose:
- and it has been 3 days or less since you should have used Sogroya, administer it as soon as you remember. Then, inject the next dose on the usual injection day.
- and it has been more than 3 days since you should have used Sogroya, skip the missed dose. Then, inject the next dose as usual, on the next scheduled day.
Do not inject an extra dose or increase the dose to make up for the missed dose.
If you stop treatment with Sogroya
Do not stop your treatment with Sogroya without consulting your doctor.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects observed in children and adolescents
Very common (may affect more than 1 in 10 people)
- Headache.
Common (may affect up to 1 in 10 people)
- Swelling in hands and feet due to fluid accumulation under the skin (peripheral edema)
- The adrenal glands do not produce enough steroid hormones (adrenal insufficiency)
- Decreased thyroid hormone (hypothyroidism)
- Redness and pain at the injection site (injection site reactions)
- Joint pain (arthralgia)
- Pain in arms or legs (pain in the extremities)
- High blood sugar level (hyperglycemia)
- Feeling very tired (fatigue).
Side effects observed in adults
Very common (may affect more than 1 in 10 people)
- Headache.
Common (may affect up to 1 in 10 people)
- The adrenal glands do not produce enough steroid hormones (adrenal insufficiency)
- Decreased thyroid hormone (hypothyroidism)
- High blood sugar level (hyperglycemia)
- Feeling of "tingling", especially in the fingers (paresthesia)
- Rash
- Hives
- Joint pain (arthralgia), muscle pain (myalgia), muscle stiffness
- Swelling in hands and feet due to fluid accumulation under the skin (peripheral edema)
- Feeling very tired or weak (fatigue or asthenia)
- Redness and pain at the injection site (injection site reactions).
Uncommon (may affect up to 1 in 100 people)
- Thickening of the skin at the injection site (lipoatrophy)
- Feeling of numbness and tingling in the hands (carpal tunnel syndrome)
- Itching (pruritus)
- Joint stiffness.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Sogroya
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the carton of the pen after “EXP”. The expiry date is the last day of the month indicated.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze. Keep away from the cooling element of the refrigerator.
After first use
Use within 6 weeks after first use. Store in a refrigerator (between 2 °C and 8 °C).
Before and after first use
If you cannot refrigerate it (for example, during travel), Sogroya can be temporarily stored at temperatures below 30 °C for a maximum total of 72 hours (3 days). Return Sogroya to the refrigerator after storing it at this temperature. If you have stored it out of the refrigerator and then put it back in the refrigerator, the total time out of refrigeration must not exceed 3 days; keep track of this carefully. Discard the Sogroya pen if it has been stored at 30 °C for more than 72 hours or above 30 °C for any period of time.
Record the time out of refrigerator:_____________
Store Sogroya in the outer packaging with the pen cap on to protect it from light.
Always remove the needle after each injection and store the pen without the needle attached.
Do not use this medicine if you notice that the solution is not transparent or slightly opalescent, colorless to slightly yellow, and is not free from visible particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Sogroya Composition
- The active substance is somapacitan. One ml of solution contains 6.7 mg of somapacitan. Each pre-filled pen contains 10 mg of somapacitan in 1.5 ml of solution.
- The other ingredients are: histidine, mannitol, poloxamer 188, phenol, water for injections, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment). Also, refer to section 2 “What you need to know before you start using Sogroya” for more information on sodium.
Product Appearance and Container Contents
Sogroya is an injectable liquid in a pre-filled pen with a transparent or slightly opalescent appearance, colorless or slightly yellow, and free of visible particles.
Sogroya 10 mg/1.5 ml solution for injection in a pre-filled pen with a yellow push button is available in the following container sizes: one container with 1 pre-filled pen or a multiple container with 5 containers, each with 1 pre-filled pen.
Only some container sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
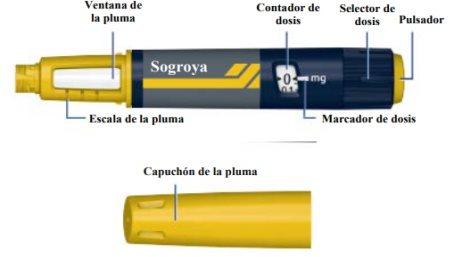
Instructions for Use Overview of the Sogroya 10mg/1.5ml Pen
Needle (Example)
| |
How to Use the Sogroya Pen Follow 5 Steps During Sogroya Injection: Step 1. Preparation of the Sogroya Pen..........................................................................................73 Step 2. Checking the Flow with Each New Pen.....................................................................74 Step 3. Dose Selection..............................................................................................................75 Step 4. Injection of the Dose........................................................................................................76 Step 5. After Injection.........................................................................................................77 For More Information on the Pen, See the Sections:Check How Much Sogroya is Left, How to Care for the Pen, Important Information. Read the leaflet and these instructions carefully before using the pre-filled Sogroya pen. Pay special attention to these notes because they are important for the safe use of the pen. Additional Information Sogroya contains 10 mg of somapacitan and can be used to inject doses from 0.05 mg to 4 mg in 0.05 mg intervals. Sogroya can only be used under the skin (subcutaneously). Needles are not included and must be obtained separately. The pre-filled Sogroya pen is designed to be used with disposable needles of 4 mm to 8 mm in length and 30 G to 32 G in thickness. Do Notshare the Sogroya pen or needles with another person. You may infect them or get an infection. Do Not Usethe pen without having received proper training from your doctor or nurse. Make sure you feel confident to inject yourself with the pen before starting treatment. If you are blind or have low vision and cannot read the dose counter on the pen, do not use this pen without help. Ask for help from a person without vision problems and trained in the use of the pen. | |
Step 1. Preparation of the Sogroya Pen | |
Make Sure You are Using the Correct Pen. Especially if you use more than one type of injectable medication. Using the wrong medication can be harmful to your health. |
|
|
|
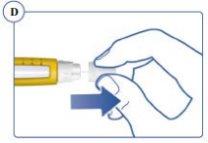
The needle is protected by two caps. You must remove both caps. If you forget to remove any, no medication will be injected. See Figures C and D. |
|
A drop of Sogroya may appear on the tip of the needle. This is normal, but you must still check the flow with each new pen. See Step 2. |
|
Always Use a New Needle for Each Injection.This reduces the risk of contamination, infection, loss of Sogroya, and needle blockage, which can lead to inaccurate dosing. Never use a bent or damaged needle. | |
Step 2. Checking the Flow with Each New Pen | |
If Your Pen is Already in Use, go to Step 3.
|
|
|
|
|
|
If Sogroya Does Not Appear, repeat Step 2 a maximum of 6 times. If no drop of Sogroya still appears, change the needle once as described in Step 5 and repeat Steps 1 and 2 again. |
|
If Sogroya does not appear when checking the flow, the needle may be blocked or damaged. Do not use the pen if Sogroya still does not appear after changing the needle. The pen may be defective. | |
Step 3. Dose Selection | |
Once you have selected the dose, proceed to Step 4. If There is Not Enough Sogroyato select a full dose, refer to Check How Much Sogroya is Left. |
|
The dose counter shows the dose in mg. See Figures J and K. Always use the dose marker to select the exact dose. Do Not Count the Pen Clicks. Do Not Use the Pen Scale (see Overview of the Sogroya Pen)to measure the amount of growth hormone to be injected. Only the dose marker will indicate the exact number of mg. |
|
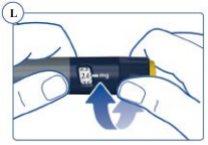
If you select an incorrect dose, you can turn the dose selector clockwise or counterclockwise to correct the dose. See Figure L. The pen clicks sound and feel different when turning the dose selector clockwise or counterclockwise or if you accidentally pass the remaining mg. |
|
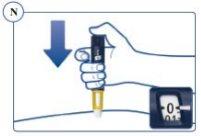
Step 4. Injection of the Dose | |
Make sure you can see the dose counter. Do Not Cover it with Your Fingers. This could block the injection. Remember to Change the Injection Site Each Week. |
|
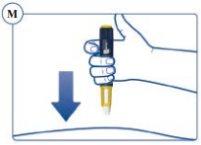
Continue to Press the Push Button with the Needle in the Skin. |
|
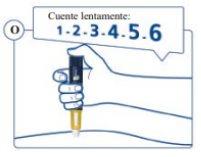
the Skin and Slowly Count to 6to ensure you administer the full dose (see Figure O). |
|
If the “0” does not appear on the dose counter after continuously pressing the push button, the needle or pen may be blocked or damaged and you may not have received any Sogroya, even though the dose counter has moved from the original dose you set. Remove the needle as described in Step 5 and repeat Steps 1 to 4. | |
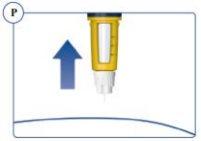
A drop of Sogroya may appear on the tip of the needle after injection. This is normal and does not |
|
Step 5. After Injection | |
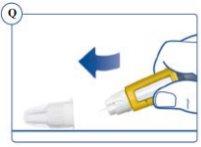
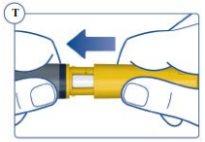
or the outer cap. See Figure Q. |
|
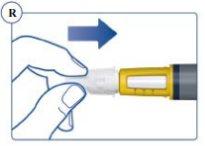
completely and carefully. See Figure R. |
|
Always Dispose of the Needle After Each Injection. When the pen is empty, remove and dispose of the needle as described above and discard the pen separately, following the instructions of your doctor, nurse, pharmacist, or local authorities. The pen cap and empty container can be thrown away in the trash. |
|
To store the pen, refer to Storage of Sogroyain this leaflet. |
|
Do Not Attempt to Put the Inner Needle Cap Back On. You could prick yourself with the needle. Always remove the needle from the pen immediately after each injection. This reduces the risk of contamination, infection, loss of Sogroya, and needle blockage, which can lead to inaccurate dosing. | |
Check How Much Sogroya is Left | |
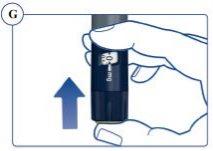
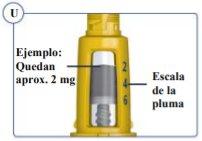
The pen scale shows the approximate amount of Sogroya left in the pen. See Figure U. |
|
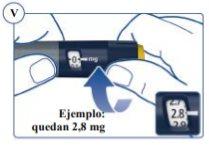
To find out how much Sogroya is left, use the dose counter: Turn the dose selector clockwise until the dose counter stops. You can select a maximum dose of 4 mg. If it shows “4”, there are at least 4 mg left in the pen. If the dose counter stops at “2.8”, there are only 2.8 mg left in the pen. See Figure V. |
|
What if I Need a Dose Larger than What is Left in the Pen? | It is not possible to select a dose larger than the amount of mg left in the pen. If you need more Sogroya than what is left in the pen, you can use a new pen or divide the dose between the pen in use and the new one. You can only divide the dose if your doctor or nurse has recommended it and you have received the relevant training. Use a calculator to plan the doses following your doctor’s or nurse’s instructions. Be very careful when doing the calculation, as it can lead to a medication error. In case of doubt about how to divide the dose into two pens, select and inject the dose you need with a new pen. |
How to Care for the Pen | |
How Should I Care for the Pen? | Be careful not to drop the pen or hit it against hard surfaces. Do not expose the pen to dust, dirt, liquids, or direct light. Do not attempt to refill the pen; it is pre-filled and must be discarded when empty. |
What if the Pen is Dropped? | If the pen is dropped or you think it is not working correctly, insert a new disposable needle and check the flow before injection, see Steps 1 and 2. If the pen has been dropped, check the cartridge; if it is cracked, do not use the pen. |
How Do I Clean the Pen? | Do not wash, soak, or lubricate the pen. It can be cleaned with a mild detergent and a damp cloth. |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SOGROYA 10 mg/1.5 ml injectable solution in a prefilled penDosage form: INJECTABLE, 15 mgActive substance: somapacitanManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 5 mgActive substance: somapacitanManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for SOGROYA 10 mg/1.5 ml injectable solution in a prefilled pen
Discuss questions about SOGROYA 10 mg/1.5 ml injectable solution in a prefilled pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













 Important Information
Important Information





