SKYRIZI 360 mg INJECTABLE SOLUTION IN CARTRIDGE


How to use SKYRIZI 360 mg INJECTABLE SOLUTION IN CARTRIDGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Skyrizi 180mg solution for injection in a cartridge
Skyrizi 360 mg solution for injection in a cartridge
risankizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Skyrizi and what is it used for
- What you need to know before you use Skyrizi
- How to use Skyrizi
- Possible side effects
- Storage of Skyrizi
- Contents of the pack and further information
1. What is Skyrizi and what is it used for
Skyrizi contains the active substance risankizumab.
Skyrizi is used to treat adult patients with:
- moderate to severe Crohn's disease
- moderate to severe ulcerative colitis
How Skyrizi works
This medicine works by blocking a protein in the body called "IL-23" that causes inflammation.
Crohn's disease
Crohn's disease is an inflammatory disease of the digestive tract. If you have active Crohn's disease, you may first be given other medicines. If these medicines do not work well enough, you will be given Skyrizi to treat your Crohn's disease.
Ulcerative colitis
Ulcerative colitis is an inflammatory disease of the large intestine. If you have active ulcerative colitis, you may first be given other medicines. If these medicines do not work well enough or if you cannot take them, you will be given Skyrizi to treat your ulcerative colitis.
Skyrizi reduces inflammation and can help reduce the signs and symptoms of your disease.
2. What you need to know before you use Skyrizi
Do not use Skyrizi
- if you are allergic to risankizumab or any of the other ingredients of this medicine (listed in section 6).
- if you have an infection that your doctor thinks is important, for example, active tuberculosis.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before and during treatment with Skyrizi:
- if you have a current infection or if you have an infection that keeps coming back.
- if you have tuberculosis (TB).
- if you have recently received or are scheduled to receive a vaccine. Certain vaccines should not be given during treatment with Skyrizi.
It is important that your doctor or nurse keeps a record of the batch number of Skyrizi.
Each time you receive a new pack of Skyrizi, your doctor or nurse should write down the date and the batch number (which appears on the pack after "Lot").
Allergic reactions
Talk to your doctor or seek medical attention immediately if you notice any signs of an allergic reaction while receiving Skyrizi, such as:
- difficulty breathing or swallowing
- swelling of the face, lips, tongue, or throat
- severe itching of the skin, with a red rash or bumps
Children and adolescents
Skyrizi is not recommended for children and adolescents under 18 years of age, as the use of Skyrizi in this age group has not been studied.
Other medicines and Skyrizi
Tell your doctor, pharmacist, or nurse:
- if you are using, have recently used, or might use any other medicines.
- if you have recently been vaccinated or are scheduled to be vaccinated. Certain vaccines should not be given during treatment with Skyrizi.
If in doubt, consult your doctor, pharmacist, or nurse before using Skyrizi and during treatment.
Pregnancy, contraception, and breastfeeding
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. You must do this because we do not know how this medicine affects the baby.
If you are a woman who can become pregnant, you must use contraception while being treated with this medicine and for at least 21 weeks after your last dose of Skyrizi.
If you are breastfeeding or plan to breastfeed, ask your doctor for advice before taking this medicine.
Driving and using machines
Skyrizi is unlikely to affect your ability to drive or use machines.
Skyrizi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial, which is essentially "sodium-free".
3. How to use Skyrizi
Follow exactly the instructions for administering this medicine given by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
This medicine is given by injection under the skin (called "subcutaneous injection").
How much Skyrizi to use
You will start treatment with Skyrizi at an initial dose that will be given to you by your doctor or nurse through a drip in your arm (intravenous infusion).
Initial doses
How much? | When? | |
Crohn's disease | 600 mg | When your doctor tells you |
600 mg | 4 weeks after the 1st dose | |
600 mg | 4 weeks after the 2nd dose |
Ulcerative colitis | How much? | When? |
1,200 mg | When your doctor tells you | |
1,200 mg | 4 weeks after the 1st dose | |
1,200 mg | 4 weeks after the 2nd dose |
Afterwards, you will receive Skyrizi by injection under the skin.
Maintenance doses
Crohn's disease | How much? | When? |
1st maintenance dose | 360 mg | 4 weeks after the last initial dose (in week 12) |
Following doses | 360 mg | Every 8 weeks, starting after the 1st maintenance dose |
Ulcerative colitis | How much? | When? |
1st maintenance dose | 180 mg or 360 mg | 4 weeks after the last initial dose (in week 12) |
Following doses | 180 mg or 360 mg | Every 8 weeks, starting after the 1st maintenance dose |
You and your doctor, pharmacist, or nurse will decide if you can inject this medicine yourself. You should not inject this medicine yourself unless your doctor, pharmacist, or nurse has taught you how to do it. It is also possible that the injection will be given by a caregiver who has learned how to do it.
Read section 7 "Instructions for use" at the end of this leaflet before giving yourself the Skyrizi injection.
If you use more Skyrizi than you should
If you have used more Skyrizi than you should or have administered the dose before it was due, talk to your doctor.
If you forget to use Skyrizi
If you forget to give yourself the Skyrizi injection, you should inject a dose as soon as you remember. If in doubt, consult your doctor.
If you stop treatment with Skyrizi
Do not stop using Skyrizi without talking to your doctor first. If you stop treatment, your symptoms may come back.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Talk to your doctor or seek medical attention immediately if you have any symptoms of a serious infection, such as:
- fever, flu-like symptoms, night sweats
- feeling tired or having trouble breathing, persistent cough
- heat, redness, and pain in the skin or a painful skin rash with blisters
Your doctor will decide if you can continue using Skyrizi.
Other side effects
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects.
Very common:may affect more than 1 in 10 people
- upper respiratory tract infections with symptoms such as sore throat and nasal congestion
Common:may affect up to 1 in 10 people
- feeling tired
- fungal skin infection
- reactions at the injection site (such as redness or pain)
- itching
- headache
- rash
- eczema
Uncommon:may affect up to 1 in 100 people
- small red bumps on the skin
- hives (urticaria)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Skyrizi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the cartridge label and on the outer carton after EXP.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
If necessary, the cartridge can also be stored at room temperature (up to 25°C) for a maximum of 24 hours.
Keep the cartridge in the original packaging to protect it from light.
Do not use this medicine if the liquid is cloudy or contains large flakes or particles.
Each body injector with cartridge is for single use only.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Skyrizi Composition
The active ingredient is risankizumab.
Skyrizi 180 mg injectable solution in a cartridge
- Each cartridge contains 180 mg of risankizumab in 1.2 ml of solution.
- The other components are sodium acetate trihydrate, acetic acid, trehalose dihydrate, polysorbate 20, and water for injectable preparations.
Skyrizi 360 mg injectable solution in a cartridge
- Each cartridge contains 360 mg of risankizumab in 2.4 ml of solution.
- The other components are sodium acetate trihydrate, acetic acid, trehalose dihydrate, polysorbate 20, and water for injectable preparations.
Product Appearance and Container Contents
Skyrizi is a clear, colorless to yellowish liquid contained in a cartridge. The liquid may contain tiny, transparent or white particles.
Each container contains 1 cartridge and 1 body injector.
Marketing Authorization Holder and Manufacturer
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Ludwigshafen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien AbbVie SA Tel: +32 10 477811 | Lithuania AbbVie UAB Tel: +370 5 205 3023 |
| Luxembourg/Luxemburg AbbVie SA Belgique/Belgien Tel: +32 10 477811 |
Czech Republic AbbVie s.r.o. Tel: +420 233 098 111 | Hungary AbbVie Kft. Tel: +36 1 455 8600 |
Denmark AbbVie A/S Tlf: +45 72 30-20-28 | Malta V.J.Salomone Pharma Limited Tel: +356 22983201 |
Germany AbbVie Deutschland GmbH & Co. KG Tel: 00800 222843 33 (toll-free) Tel: +49 (0) 611 / 1720-0 | Netherlands AbbVie B.V. Tel: +31 (0)88 322 2843 |
Estonia AbbVie OÜ Tel: +372 623 1011 | Norway AbbVie AS Tlf: +47 67 81 80 00 |
Greece AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Tel: +30 214 4165 555 | Austria AbbVie GmbH Tel: +43 1 20589-0 |
Spain AbbVie Spain, S.L.U. Tel: +34 91 384 09 10 | Poland AbbVie Sp. z o.o. Tel: +48 22 372 78 00 |
France AbbVie Tel: +33 (0) 1 45 60 13 00 | Portugal AbbVie, Lda. Tel: +351 (0)21 1908400 |
Croatia AbbVie d.o.o. Tel: +385 (0)1 5625 501 | Romania AbbVie S.R.L. Tel: +40 21 529 30 35 |
Ireland AbbVie Limited Tel: +353 (0)1 4287900 | Slovenia AbbVie Biofarmacevtska družba d.o.o. Tel: +386 (1)32 08 060 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia AbbVie s.r.o. Tel: +421 2 5050 0777 |
Italy AbbVie S.r.l. Tel: +39 06 928921 | Finland AbbVie Oy Tel: +358 (0)10 2411 200 |
Cyprus Lifepharma (Z.A.M.) Ltd Tel: +357 22 34 74 40 | Sweden AbbVie AB Tel: +46 (0)8 684 44 600 |
Latvia AbbVie SIA Tel: +371 67605000 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
Up-to-date information on this product is available below or on the outer packaging by scanning the QR code using a smartphone. The same information is also available on the following website: www.skyrizi.eu
QR Code to be included
To request a copy of this leaflet in
- Instructions for Use
Read the entire Section 7 before using Skyrizi
Skyrizi Body Injector
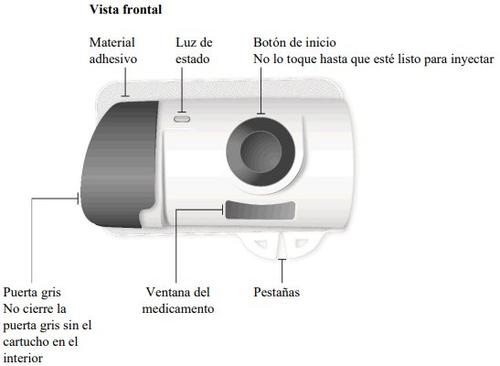
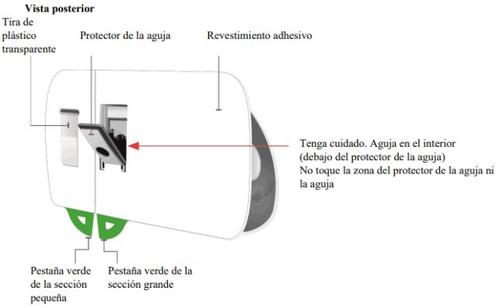
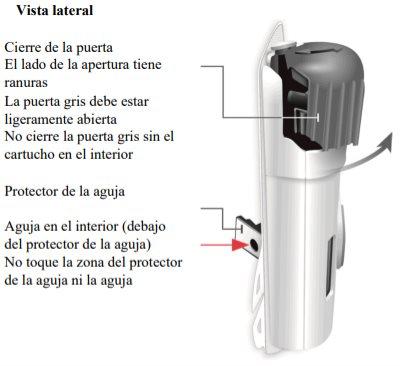
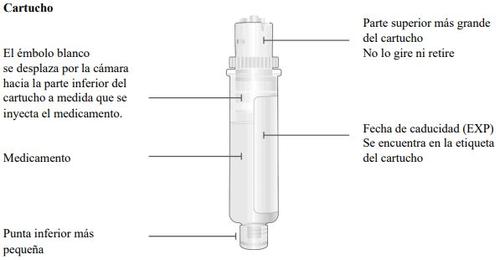
Important Information to Know Before Injecting Skyrizi
- You must have received training on how to inject Skyrizi before administering an injection. If you need help, consult your doctor, pharmacist, or nurse.
- Mark the dates on a calendar to know when it's time to inject Skyrizi.
- The single-use body injector is designed for use only with the Skyrizi cartridge.
- Keep Skyrizi in its original packaging to protect the medication from light until the time of use.
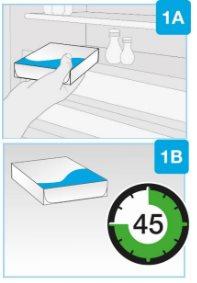
- Remove the box from the refrigerator and let it sit at room temperature, away from direct sunlight, for at least 45 and up to 90 minutesbefore injection.
- Do notget the body injector wet with water or any other liquid.
- Do nottouch the start button until you have placed the loaded body injector on your skin and are ready to inject.
- You can only press the start button once.
- Limit physical activity during the injection process. You can perform moderate physical activities, such as walking, stretching, or bending.
- Do notdelay the injection of the medication once the clean cartridge is loaded into the body injector. If you wait, the medication will dry out, and the body injector will stop working.
- Do notinject the medication if the liquid in the inspection window is cloudy or contains flakes or large particles. The liquid should be clear to yellowish and may contain tiny, transparent or white particles.
- Do notshake the box, cartridge, or body injector.
- Do notreuse the cartridge or body injector.
Return this medication to the pharmacy
- after the expiration date (EXP) indicated
- if the liquid has been frozen at any time (even if it has been thawed)
- if the cartridge or body injector has been dropped or damaged
- if the perforations on the box are broken
- if the white paper cover of the tray is missing or broken
Follow these steps each time you use Skyrizi
STEP 1: Prepare | ||
| Remove the box from the refrigerator and let it sit at room temperature, away from direct sunlight, for at least 45 and up to 90 minutesbefore injection.
| |
Special waste container
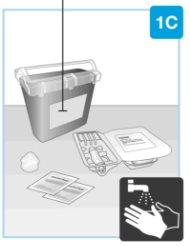
| Gather all materials and wash your hands On a smooth and clean surface, place the following:
Wash and dry your hands. | |
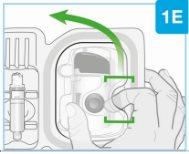
| Remove the paper seal from the tray
| |
| Lift the plastic cover
| |
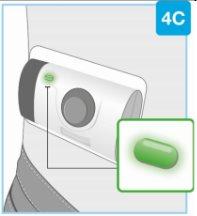
Needle inside (under the needle protector) | Door gray Start button | Inspect the body injector
If the gray start button is pressed before placing the injector on the body, the body injector will no longer be usable. If this happens, inform your doctor, pharmacist, or nurse. |
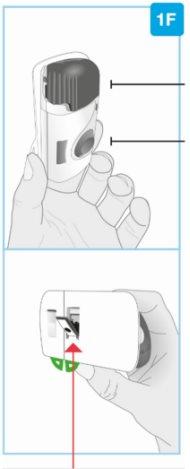
STEP 2: Prepare the Body Injector | ||
Gray door
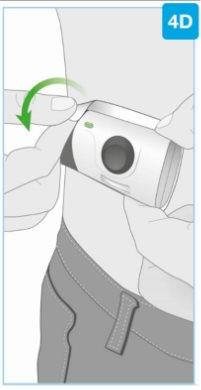
Needle protector | Rear view Needle inside (under the needle protector) | Open the gray door all the way
Set the body injector aside. |
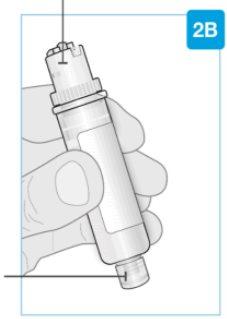
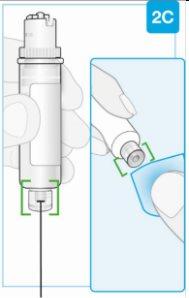
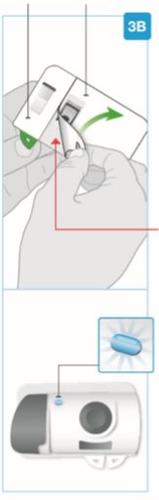
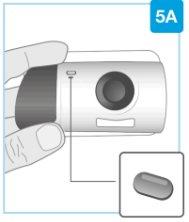
Top part of the cartridge (larger) | Inspect the Cartridge Carefully remove the cartridge from the plastic tray.
Check the Cartridge
| |
Bottom part of the cartridge (smaller) |
| |
Bottom part of the cartridge (smaller) | Clean the bottom part of the cartridge Locate the bottom part of the cartridge.
| |
Clean the center of the bottom part (smaller) | ||
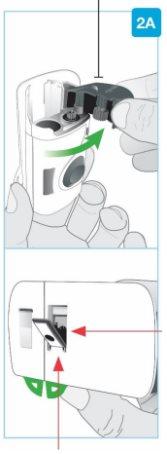
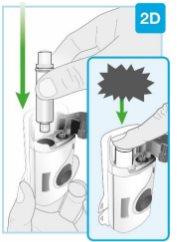
Insert straight "click"
| Load the clean cartridge into the body injector
Make sure to proceed with the next step immediately. If you wait, the medication will dry out. | |
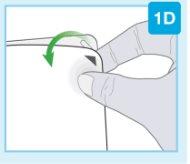
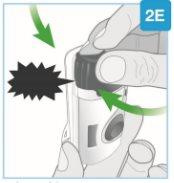
"snap" | Close the gray door Turn the gray door to the left, then press firmly and listen for a "snap" as the gray door closes.
| |
STEP 3: Prepare the Injection | ||
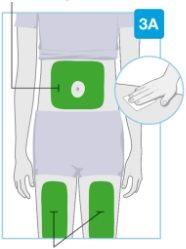
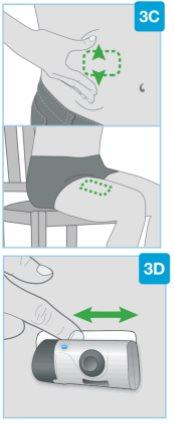
Injection sites
Injection sites | Choose and clean the injection site Choose one of these 3 areas for injection:
Do notinject into skin areas with folds or natural protrusions, as the body injector may fall off during use. Before injection, clean the injection site with an alcohol swab using circular motions.
| |
Small section Large section
| Needle inside (under the needle protector) | Activated Injector The status light flashes blue. |
| If the status light flashes red, the body injector is not working properly. Do not continue using it. Consult your doctor, pharmacist, or nurse for help. If the body injector is stuck to your body, carefully remove it from the skin. | |
|
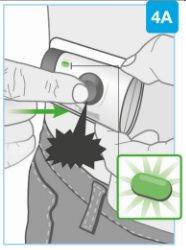
Injection in the abdomen and thigh marked with green dots, arrows indicate movement of a cylindrical applicator device"> | Prepare the body injector for placement
Make sure to place the body injector so that you can see the blue status light. Place the body injector on the skin
Proceed immediately to the next step. |
STEP 4: Skyrizi Injection | |
"click" | Start the injection Press the gray start button firmly and release it
Do notcontinue using the body injector if the status light blinks red. Remove it carefully from the skin if the status light blinks red. If this occurs, inform your doctor, pharmacist, or nurse. |
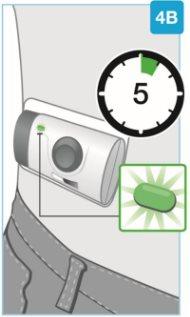
| Wait for the injection to finish
Do notcontinue using the body injector if the status light blinks red. Remove it carefully from the skin if the status light blinks red. If this occurs, inform your doctor, pharmacist, or nurse. |
| The injection is complete when:
|
| Remove the body injector
Proceed to the next step. |
STEP 5: Completion | |
| Check the body injector Inspect the medication window and the status light. Check that the white plunger fills the entire medication window and that the green light turns off, indicating that all the medication has been injected.
|
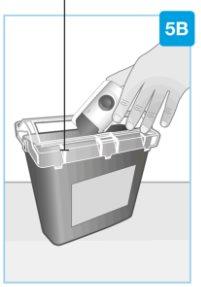
Special waste container
| Disposal Discard the used body injector in a special waste container immediately after use.
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SKYRIZI 360 mg INJECTABLE SOLUTION IN CARTRIDGEDosage form: INJECTABLE, 150 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: INJECTABLE, 150 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: INJECTABLE, 180 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription required
Online doctors for SKYRIZI 360 mg INJECTABLE SOLUTION IN CARTRIDGE
Discuss questions about SKYRIZI 360 mg INJECTABLE SOLUTION IN CARTRIDGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions