
SIGNIFOR 40 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION

How to use SIGNIFOR 40 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Signifor 10mg powder and solvent for injectable suspension
Signifor 20mg powder and solvent for injectable suspension
Signifor 30mg powder and solvent for injectable suspension
Signifor 40mg powder and solvent for injectable suspension
Signifor 60mg powder and solvent for injectable suspension
pasireotide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, nurse or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Signifor and what is it used for
- What you need to know before you use Signifor
- How to use Signifor
- Possible side effects
- Storage of Signifor
- Contents of the pack and other information
1. What is Signifor and what is it used for
Signifor is a medicine that contains the active substance pasireotide. It is used to treat acromegaly in adult patients. It is also used to treat Cushing's disease in adult patients for whom surgery is not an option or for whom surgery has failed.
Acromegaly
Acromegaly is caused by a type of tumor called a pituitary adenoma, which occurs in the pituitary gland at the base of the brain. The adenoma causes the body to produce too much of the hormones that control the growth of tissues, organs, or bones, resulting in an increase in the size of bones and tissues, especially in the hands and feet.
Signifor reduces the production of these hormones and may also reduce the size of the adenoma. As a result, it reduces the symptoms of acromegaly, which include headache, increased sweating, numbness of the hands and feet, fatigue, and joint pain.
Cushing's disease
Cushing's disease is caused by an increase in the size of the pituitary gland (a gland located at the base of the brain) called a pituitary adenoma. This causes the body to produce too much of a hormone called adrenocorticotropic hormone (ACTH), which in turn causes an increase in the production of another hormone called cortisol.
The human body naturally produces a substance called somatostatin, which blocks the production of certain hormones, including ACTH. Pasireotide works in a very similar way to somatostatin. Signifor is therefore able to block the production of ACTH, helping to control the overproduction of cortisol and improve the symptoms of Cushing's disease.
If you have any questions about how Signifor works or why you have been prescribed this medicine, ask your doctor.
2. What you need to know before you use Signifor
Do not use Signifor:
- if you are allergic to pasireotide or any of the other ingredients of this medicine (listed in section 6).
- if you have severe liver problems.
Warnings and precautions
Tell your doctor before you start using Signifor if you have or have ever had:
- problems with blood sugar levels, either too high (as in hyperglycemia/diabetes) or too low (hypoglycemia);
- heart problems such as a recent heart attack, congestive heart failure (a type of heart disease where the heart cannot pump enough blood through the body) or sudden and severe chest pain (usually felt as pressure, heaviness, tightness, or pain in the chest);
- a heart rhythm disorder, such as an irregular heartbeat or an abnormal electrical signal called "prolonged QT interval" or "QT prolongation";
- low levels of potassium or magnesium in the blood;
- gallstones;
- or if you are taking anticoagulants (medicines used to reduce the blood's ability to clot), your doctor will monitor your coagulation parameters and may adjust your anticoagulant dose.
During your treatment with Signifor
- Signifor may cause an increase in blood sugar. Your doctor may monitor your blood sugar level and start treatment or adjust your antidiabetic medicine.
- Signifor controls the overproduction of cortisol. The control may be too strong and you may experience signs or symptoms associated with a lack of cortisol, such as extreme weakness, fatigue, weight loss, nausea, vomiting, or low blood pressure. If this happens, tell your doctor immediately.
- Signifor may reduce your heart rate. Your doctor may monitor your heart rate using a machine that measures the electrical activity of the heart (an "ECG" or electrocardiogram). If you are using a medicine to treat a heart problem, your doctor may also need to adjust your dose.
- your doctor may also periodically monitor your gallbladder, liver enzymes, and pituitary hormones, as they may be affected by this medicine.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age because there is no data available for this age group.
Other medicines and Signifor
Signifor may affect the way other medicines work. If you are using other medicines at the same time as Signifor (including non-prescription medicines), your doctor may need to monitor your heart more closely or change the dose of Signifor or the other medicines. Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. In particular, tell your doctor if you are using:
- medicines used in organ transplantation to reduce the activity of the immune system (cyclosporine);
- medicines to treat high blood sugar levels (such as in diabetes) or low blood sugar levels (hypoglycemia), such as:
- insulin
- metformin, liraglutide, vildagliptin, nateglinide (antidiabetic medicines);
- medicines to treat irregular heart rhythm, such as those containing disopyramide, procainamide, quinidine, sotalol, dofetilide, ibutilide, amiodarone, or dronedarone;
- medicines to treat bacterial infections (by mouth: clarithromycin, moxifloxacin; by injection: erythromycin, pentamidine);
- medicines to treat fungal infections (ketoconazole, except in shampoo);
- medicines to treat certain psychiatric disorders (chlorpromazine, thioridazine, fluphenazine, pimozide, haloperidol, tiapride, amisulpride, sertindol, methadone);
- medicines to treat hay fever and other allergies (terfenadine, astemizole, mizolastine);
- medicines used to prevent or treat malaria (chloroquine, halofantrine, lumefantrine);
- medicines to control blood pressure, such as:
- beta blockers (metoprolol, carteolol, propranolol, sotalol)
- calcium channel blockers (bepridil, verapamil, diltiazem)
- cholinesterase inhibitors (rivastigmine, physostigmine);
- medicines to control the balance of electrolytes (potassium, magnesium) in the body.
Pregnancy, breastfeeding, and fertility
Ask your doctor or pharmacist for advice before taking any medicine.
- Do not use Signifor during pregnancy unless clearly necessary. If you are pregnant or think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
- If you are breastfeeding, ask your doctor for advice before taking this medicine, as it is not known whether Signifor passes into breast milk.
- If you are a sexually active woman, you should use an effective method of contraception during treatment. Ask your doctor about the need for contraception before using this medicine.
Driving and using machines
Signifor may have a minor effect on your ability to drive and use machines, as some of the side effects you may experience while using Signifor, such as headache, dizziness, and fatigue, may reduce your ability to drive and use machines safely.
Important information about some of the ingredients of Signifor
Signifor contains less than 1 mmol of sodium (23 mg) per dose; i.e., it is essentially "sodium-free".
3. How to use Signifor
This medicine will only be administered by a trained healthcare professional.
How much Signifor to use
Acromegaly
The recommended starting dose of Signifor for acromegaly is 40 mg every 4 weeks. After starting treatment, your doctor may reassess your dose. This may involve measuring the levels of growth hormone or other hormones in your body. Depending on the results and how you feel, it may be necessary to reduce or increase the dose of Signifor administered in each injection. The dose should not exceed 60 mg. If you have liver disease before starting treatment with Signifor, your doctor may decide to start treatment with a dose of 20 mg.
Cushing's disease
The normal starting dose of Signifor for Cushing's disease is 10 mg every 4 weeks. After starting treatment, your doctor may review the dose. This may involve measuring the levels of cortisol in the blood or urine. Depending on the results and how you feel, it may be necessary to reduce or increase the dose of Signifor administered in each injection. The dose should not exceed 40 mg.
Your doctor will regularly monitor how you respond to treatment with Signifor and decide what dose is best for you.
How to use Signifor
Your doctor or nurse will inject Signifor. If you have any questions, ask your doctor, nurse, or pharmacist.
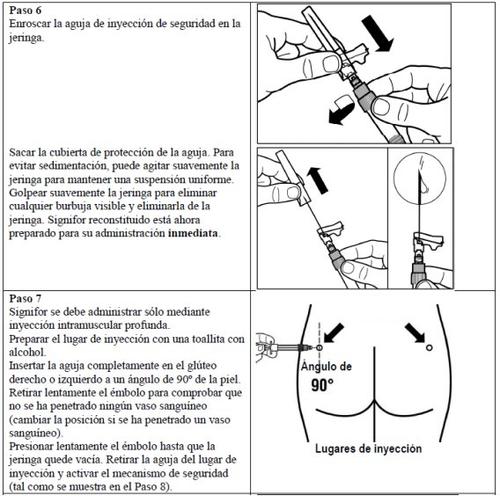
Signifor is used by intramuscular injection. This means it is injected through a needle into the muscles of your buttocks.
For how long to use Signifor
This is a long-term treatment, possibly lasting years. Your doctor will regularly monitor your disease to check that the treatment is having the desired effect. Your treatment with Signifor should continue as long as your doctor tells you it is necessary.
If you stop treatment with Signifor
If you stop your treatment with Signifor, your symptoms may come back. Therefore, do not stop your treatment with Signifor unless your doctor tells you to.
If you have any other questions about the use of this medicine, ask your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious. Tell your doctor immediately if you experience any of the following:
Very common (may affect more than 1 in 10 people)
- High blood sugar level. You may notice excessive thirst, urination, increased appetite with weight loss, fatigue, nausea, vomiting, abdominal pain.
- Gallstones or associated complications. You may experience fever, chills, yellowing of the skin/eyes, sudden back pain or pain in the right side of the abdomen.
Common (may affect up to 1 in 10 people)
- Low levels of cortisol. You may experience extreme weakness, fatigue, weight loss, nausea, vomiting, and low blood pressure.
- Slow heart rate.
- Prolonged QT interval (an abnormal electrical signal in your heart that can be seen on tests).
- Bile flow problems (cholestasis). You may experience yellowing of the skin, dark urine, pale stools, and itching.
- Inflammation of the gallbladder (cholecystitis).
Other side effects of Signifor may include:
Very common (may affect more than 1 in 10 people)
- Diarrhea
- Nausea
- Abdominal pain
- Fatigue
Common (may affect up to 1 in 10 people)
- Fatigue, weakness, pale skin (signs of low red blood cell count)
- Loss of appetite
- Headache
- Swelling
- Vomiting
- Dizziness
- Pain, discomfort, itching, and swelling at the injection site
- Changes in liver function test results
- Abnormal blood test results (sign of elevated creatine phosphokinase, hemoglobin A1c, lipase in the blood)
- Hair loss
Uncommon (may affect up to 1 in 100 people)
- Changes in blood test results of pancreatic function (amylase)
- Abnormal blood coagulation properties
Frequency not known (cannot be estimated from the available data)
- High levels of ketone bodies (a group of substances produced in the liver) in the urine or blood (diabetic ketoacidosis) as a complication of high blood sugar. You may experience fruity breath odor, breathing problems, and confusion.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Signifor
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton, vial, and pre-filled syringe after "EXP"/"CAD". The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C). Do not freeze.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition of Signifor
- The active substance is pasireotide.
Signifor 10 mg: each vial contains 10 mg of pasireotide (as pasireotide pamoate)
Signifor 20 mg: each vial contains 20 mg of pasireotide (as pasireotide pamoate)
Signifor 30 mg: each vial contains 30 mg of pasireotide (as pasireotide pamoate)
Signifor 40 mg: each vial contains 40 mg of pasireotide (as pasireotide pamoate)
Signifor 60 mg: each vial contains 60 mg of pasireotide (as pasireotide pamoate)
- The other components are:
- In the powder: poly(D,L-lactide-co-glycolide) (50-60:40.50), poly(D,L-lactide-co-glycolide) (50:50).
- In the solvent: sodium carmellose, mannitol, poloxamer 188, water for injectable preparations.
Appearance of Signifor and Container Contents
Signifor powder is a slightly yellowish to yellowish powder in a vial. The solvent is a clear, colorless to slightly yellow or slightly brown solution in a pre-filled syringe.
Signifor 10 mg is available in single-unit containers that contain a vial of powder with 10 mg of pasireotide and a pre-filled syringe with 2 ml of solvent.
Signifor 20 mg is available in single-unit containers that contain a vial of powder with 20 mg of pasireotide and a pre-filled syringe with 2 ml of solvent.
Signifor 30 mg is available in single-unit containers that contain a vial of powder with 30 mg of pasireotide and a pre-filled syringe with 2 ml of solvent.
Signifor 40 mg is available in single-unit containers that contain a vial of powder with 40 mg of pasireotide and a pre-filled syringe with 2 ml of solvent.
Signifor 60 mg is available in single-unit containers that contain a vial of powder with 60 mg of pasireotide and a pre-filled syringe with 2 ml of solvent.
Each single-unit container contains the vial and the pre-filled syringe in a sealed blister tray with a vial adapter and a safety injection needle.
Signifor 40 mg and Signifor 60 mg are also available in multiple-unit containers that contain 3 intermediate containers.
In your country, only some doses and container sizes may be marketed.
Marketing Authorization Holder
Recordati Rare Diseases
Immeuble Le Wilson
70 avenue du Général de Gaulle
92800 Puteaux
France
Manufacturer
Recordati Rare Diseases
Immeuble Le Wilson
70 avenue du Général de Gaulle
92800 Puteaux
France
Recordati Rare Diseases
Eco River Parc
30 rue des Peupliers
92000 Nanterre
France
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Recordati Tel: +32 2 46101 36 | Lithuania Recordati AB. Tel: +46 8 545 80 230 Sweden |
Greece Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Luxembourg Recordati Tel: +32 2 46101 36 Belgium |
Czech Republic Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Hungary Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Denmark Recordati AB. Tel: +46 8 545 80 230 Sweden | Malta Recordati Rare Diseases Tel: +33 1 47 73 64 58 France |
Germany Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 | Netherlands Recordati Tel: +32 2 46101 36 Belgium |
Estonia Recordati AB. Tel: +46 8 545 80 230 Sweden | Norway Recordati AB. Tel: +46 8 545 80 230 Sweden |
Greece Recordati Hellas Tel: +30 210 6773822 | Austria Recordati Rare Diseases Germany GmbH Tel: +49 731 140 554 0 Germany |
Spain Recordati Rare Diseases Spain S.L.U. Tel: +34 91 659 28 90 | Poland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
France Recordati Rare Disease s Tel: +33 (0)1 47 73 64 58 | Portugal Jaba Recordati S.A. Tel: +351 21 432 95 00 |
Croatia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Romania Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Ireland Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France | Slovenia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Iceland Recordati AB. Tel: +46 8 545 80 230 Sweden | Slovakia Recordati Rare Diseases Tel: +33 (0)1 47 73 64 58 France |
Italy Recordati Rare Diseases Italy Srl Tel: +39 02 487 87 173 | Finland Recordati AB. Tel: +46 8 545 80 230 Sweden |
Cyprus Recordati Rare Diseases Tel: +33 1 47 73 64 58 France | Sweden Recordati AB. Tel: +46 8 545 80 230 |
Latvia Recordati AB. Tel: +46 8 545 80 230 Sweden | United Kingdom (Northern Ireland) Recordati Rare Diseases UK Ltd. Tel: +44 (0)1491 414333 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
This information is intended only for healthcare professionals:
INSTRUCTIONS FOR USE OF SIGNIFOR POWDER AND SOLVENT FOR INJECTABLE SUSPENSION
FOR INTRAMUSCULAR INJECTION ONLY
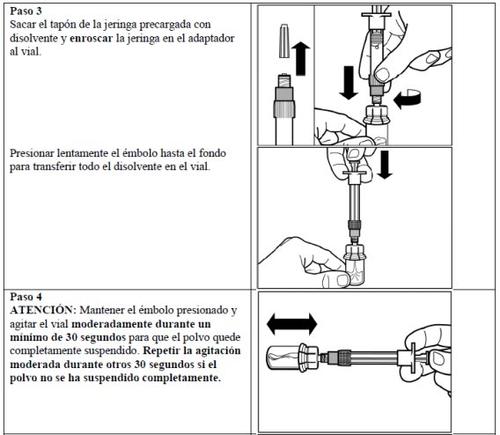
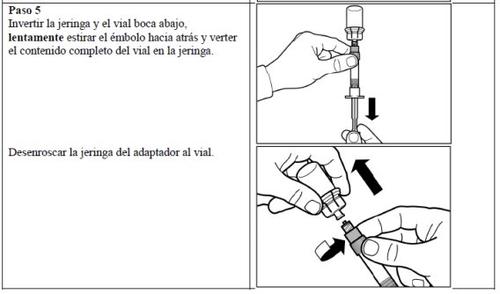
ATTENTION: In the process of reconstituting Signifor, there are two critical steps. If these are not performed correctly, this may result in the injection not being administered correctly.
|
The injection kit includes:
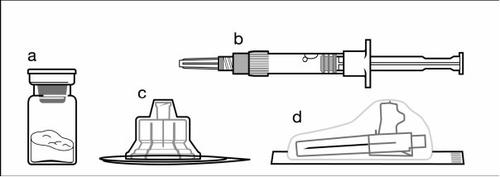
a A vial containing the powder
b A pre-filled syringe containing the solvent
c A vial adapter for reconstitution of the medicinal product
d A safety injection needle (20G x 1.5”)
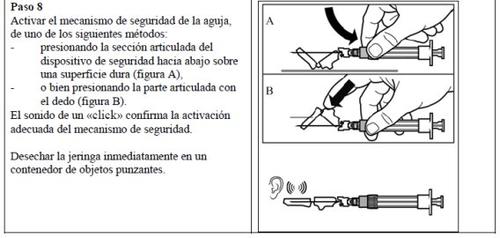
Follow the detailed instructions below to ensure the correct reconstitution of Signifor powder and solvent for injectable suspension before intramuscular injection.
Signifor suspension should only be prepared immediately before administration.
Signifor should only be administered by a healthcare professional with experience.

- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SIGNIFOR 40 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSIONDosage form: INJECTABLE, 0.3 mgActive substance: pasireotideManufacturer: Recordati Rare DiseasesPrescription requiredDosage form: INJECTABLE, 0.3 mgActive substance: pasireotideManufacturer: Recordati Rare DiseasesPrescription requiredDosage form: INJECTABLE, 0.6 mgActive substance: pasireotideManufacturer: Recordati Rare DiseasesPrescription required
Online doctors for SIGNIFOR 40 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION
Discuss questions about SIGNIFOR 40 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






