ROTARIX ORAL SUSPENSION IN PRE-FILLED ORAL APPLICATOR. LIVE ANTIRROTAVIRUS VACCINE

How to use ROTARIX ORAL SUSPENSION IN PRE-FILLED ORAL APPLICATOR. LIVE ANTIRROTAVIRUS VACCINE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Rotarix oral suspension in a pre-filled oral applicator
live attenuated rotavirus vaccine
Read all of this leaflet carefully before your child is given this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed for your child. Do not pass it on to others.
- If your child gets any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Rotarix and what is it used for
- What you need to know before your child is given Rotarix
- How Rotarix is given
- Possible side effects
- Storage of Rotarix
- Contents of the pack and other information
1. What is Rotarix and what is it used for
Rotarix is a vaccine that contains live, attenuated human rotaviruses, which helps to protect your child from the age of 6 weeks against gastroenteritis (diarrhea and vomiting) caused by rotavirus infection.
How Rotarix works
Rotavirus infection is the most common cause of severe diarrhea in infants and young children. Rotavirus is easily spread by hand-to-mouth contact with the feces of an infected person. Most children with rotavirus diarrhea recover on their own. However, some children become very ill with a severe case of vomiting, diarrhea, and loss of fluids that can be life-threatening and require hospitalization.
When the vaccine is given to a person, the immune system (the body's natural defense) will produce antibodies against the most common types of rotavirus. These antibodies protect against disease caused by these types of rotavirus.
Like all vaccines, Rotarix may not completely prevent all vaccinated individuals from getting rotavirus infections that it is intended to protect against.
2. What you need to know before your child is given Rotarix
Rotarix must not be given
- if your child has had an allergic reaction to rotavirus vaccines or to any of the other components of this vaccine (listed in section 6). The signs of an allergic reaction may include skin rash with itching, difficulty breathing, and swelling of the face or tongue.
- if your child has had a previous intussusception (a blockage of the intestine where one part of the intestine slides into another).
- if your child was born with a malformation of the intestine that could lead to an intussusception.
- if your child has a rare hereditary disease that affects the immune system called severe combined immunodeficiency (SCID).
- if your child has a severe infection with high fever. It may be necessary to postpone vaccination until your child has recovered. A minor infection, such as a cold, should not be a problem for vaccination, but tell your doctor first.
- if your child has diarrhea or vomiting. It may be necessary to postpone vaccination until your child has recovered.
Warnings and precautions
Tell your doctor or healthcare professional before your child is given Rotarix if:
- your child is in close contact with someone, such as a family member, who has a weakened immune system, e.g. a person with cancer or taking medicines that can weaken the immune system.
- your child has any problems with the gastrointestinal system.
- your child has not gained weight or grown as expected.
- your child has any disease or is taking any medicine that reduces resistance to infection, or if the mother took any medicine during pregnancy that may weaken the immune system.
If after receiving Rotarix your child experiences severe stomach pain, persistent vomiting, blood in the stool, swollen abdomen and/or high fever, contact a doctor/healthcare professional immediately (see also section 4 "Possible side effects").
As always, please take the precaution of washing your hands thoroughly after changing used diapers.
Using Rotarix with other medicines
Tell your doctor if your child is using, has recently used, or might use any other medicines or has recently received any other vaccine.
Rotarix can be given at the same time as your child is given other recommended vaccines, such as diphtheria, tetanus, pertussis (whooping cough), Haemophilus influenzaetype b, oral or inactivated poliomyelitis, hepatitis B, as well as conjugated pneumococcal and meningococcal serogroup C vaccines.
Using Rotarix with food and drinks
There are no restrictions on the consumption of food or liquids by your child, including breastfeeding, either before or after vaccination.
Breastfeeding
Based on evidence from clinical trials, breastfeeding does not reduce the protection against rotavirus gastroenteritis provided by Rotarix. Therefore, breastfeeding can continue during the vaccination period.
Rotarix contains sucrose, glucose, phenylalanine, and sodium
If your doctor has told you that your child has an intolerance to some sugars, talk to your doctor before your child is given this vaccine.
This vaccine contains 0.15 micrograms of phenylalanine per dose. Phenylalanine may be harmful in case your child has phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body cannot eliminate it properly.
This vaccine contains 34 mg of sodium (a major component of table/cooking salt) per dose.
3. How Rotarix is given
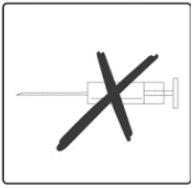
The doctor or nurse will give your child the recommended dose of Rotarix. The vaccine (1.5 ml of liquid) will be given orally. Under no circumstances should this vaccine be given by injection.
Your child will receive two doses of the vaccine. Each dose will be given separately with an interval of at least 4 weeks between the two doses. The first dose will be given from the age of 6 weeks. Both doses of the vaccine should have been given by the age of 24 weeks, although they should preferably be given before the age of 16 weeks.
Rotarix can be given to premature infants following the same vaccination schedule as for other infants, provided that gestation has lasted at least 27 weeks.
In the event that your child spits or regurgitates most of the vaccine dose, a single replacement dose can be given at the same visit.
When your child is given a first dose with Rotarix, it is recommended that your child also receives the second dose with Rotarix (and not with another rotavirus vaccine).
It is important that you follow the instructions given by your doctor or nurse regarding follow-up visits. If you miss a visit to your doctor, talk to your doctor.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
The following side effects may occur with this vaccine:
- Common (may occur in up to 1 in 10 doses of the vaccine):
- diarrhea
- irritability
- Uncommon (may occur in up to 1 in 100 doses of the vaccine):
- abdominal pain (see also the next paragraph for signs of the rare side effect intussusception)
- flatulence
- skin inflammation
Side effects that have been reported during the marketing of Rotarix include:
- Very rare: hives (urticaria)
- Very rare: intussusception (part of the intestine becomes blocked or twisted). The signs can include severe stomach pain, persistent vomiting, blood in the stool, swollen abdomen, and/or high fever. Contact a doctor/healthcare professional immediately if your child experiences any of these symptoms
- blood in stool
- children with a rare hereditary disease called severe combined immunodeficiency (SCID) may experience stomach or intestinal inflammation (gastroenteritis) and may shed the vaccine virus in their stool. The signs of gastroenteritis can include nausea, vomiting, stomach cramps, or diarrhea
Reporting of side effects
If your child experiences any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Rotarix
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the carton. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Store in the original package to protect from light.
Once opened, the vaccine should be used immediately.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Rotarix contains
- The active substance is:
Human rotavirus strain RIX4414 (live, attenuated)* | not less than 10^6.0 CCID50 |
- Produced in Vero cells
- The other components of Rotarix are: sucrose, disodium adipate, Modified Dulbecco's Eagle Medium (containing phenylalanine, sodium, glucose, and other substances), sterile water (see also section 2, "Rotarix contains sucrose, glucose, phenylalanine, and sodium").
Appearance and packaging
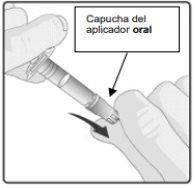
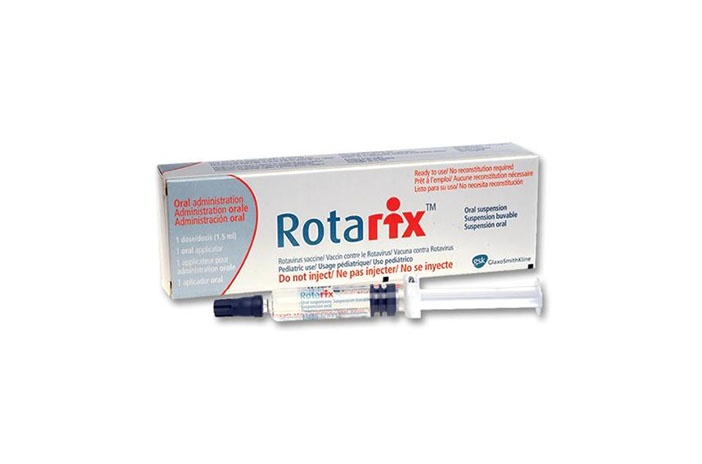
Oral suspension in a pre-filled oral applicator.
Rotarix is supplied as a clear, colorless liquid in a pre-filled oral applicator (1.5 ml).
Rotarix is available in packs of 1, 5, 10, or 25.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
GlaxoSmithKline Biologicals SA
Rue de l’Institut 89
B-1330 Rixensart
Belgium
For further information about this medicine, contact the local representative of the marketing authorization holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals SA/NV Tél/Tel: + 32 10 85 52 00 | Lietuva GlaxoSmithKline Biologicals SA Tel: +370 80000334 |
GlaxoSmithKline Biologicals SA Тел: +359 80018205 | Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals SA/NV Tél/Tel: + 32 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Magyarország GlaxoSmithKline Biologicals SA Tel.: +36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Biologicals SA Tel: +356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel: + 49 (0)89 360448701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti GlaxoSmithKline Biologicals SA Tel: +372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλάδα GlaxoSmithKline Μονοπρόσωπη A.E.B.E Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH. Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (22) 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0) 1 39 17 84 44 | Portugal Smith Kline & French Portuguesa - Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Hrvatska GlaxoSmithKline Biologicals SA Tel.: +385 800787089 | România GlaxoSmithKline Biologicals SA Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Ltd Tel: + 353 (0)1 4955000 | Slovenija GlaxoSmithKline Biologicals SA Tel: +386 80688869 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika GlaxoSmithKline Biologicals SA Tel: +421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel:+ 39 (0)45 7741 111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 10 30 30 30 |
Κύπρος GlaxoSmithKline Biologicals SA Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GlaxoSmithKline Biologicals SA Tel: +371 80205045 | United Kingdom (Northern Ireland) GlaxoSmithKline Biologicals SA Tel: + 44 (0)800 221 441 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site http://www.ema.europa.eu
This information is intended only for healthcare professionals:
The vaccine is presented as a clear, colorless liquid, free from visible particles, for oral administration.
The vaccine is ready for use (no reconstitution or dilution is required).
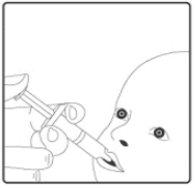
The vaccine is given orally, without being mixed with other vaccines or solutions.
The vaccine should be inspected visually for any foreign particles and/or variation in physical appearance. If any of these circumstances are observed, the vaccine should be discarded.
Disposal of unused vaccines and all materials that have come into contact with them should be done in accordance with local regulations.
Instructions for administration of the vaccine:
|
|
|
|
|
|
Discard the empty oral applicator and cap in approved biological waste containers in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROTARIX ORAL SUSPENSION IN PRE-FILLED ORAL APPLICATOR. LIVE ANTIRROTAVIRUS VACCINEDosage form: ORAL SOLUTION/SUSPENSION, 10 million germsActive substance: rota virus, live attenuatedManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 10 MILLION GERMSActive substance: rota virus, live attenuatedManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, UnknownActive substance: rota virus, pentavalent, live, reassortedManufacturer: Merck Sharp & Dohme B.V.Prescription required
Online doctors for ROTARIX ORAL SUSPENSION IN PRE-FILLED ORAL APPLICATOR. LIVE ANTIRROTAVIRUS VACCINE
Discuss questions about ROTARIX ORAL SUSPENSION IN PRE-FILLED ORAL APPLICATOR. LIVE ANTIRROTAVIRUS VACCINE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions