
ROSALGIN PRONTO 140 mg VAGINAL SOLUTION

How to use ROSALGIN PRONTO 140 mg VAGINAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Rosalgin pronto 140 mg vaginal solution
bencidamine hydrochloride
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions of the medication contained in this leaflet or as indicated by your pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 5 days.
Contents of the leaflet
- What Rosalgin pronto is and what it is used for
- What you need to know before starting to use Rosalgin pronto
- How to use Rosalgin pronto
- Possible side effects
- Storage of Rosalgin pronto
- Contents of the package and additional information
1. What Rosalgin pronto is and what it is used for
It belongs to a group of medications called anti-inflammatory agents for vaginal administration.
This medication is indicated for local and temporary relief of itching and burning, complementary to the treatment of vaginitis, in adults.
You should consult a doctor if it worsens or does not improve after 5 days.
2. What you need to know before starting to use Rosalgin pronto
Do not use Rosalgin pronto
- If you are allergic to bencidamine or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Rosalgin pronto.
- This medication is used only vaginally.
- Once the solution is applied, it is recommended to wash your hands with plenty of water, as cases of photosensitive allergic reactions have been described after exposure of the hands to sunlight without previously removing the product (see section 4).
- In case of sensitivity or irritation, or persistent burning or itching, you should discontinue treatment and consult your doctor (see section 4).
- If you notice an increase in vaginal discharge or changes in its appearance or odor or bleeding, you should consult your doctor.
Other medications and Rosalgin pronto
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
No interactions with other medications are known.
Using Rosalgin pronto with food, drinks, and alcohol
This does not apply as it is a medication for vaginal use.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
You should not use Rosalgin pronto during pregnancy unless it is clearly necessary and your doctor advises you to do so. If you need treatment, you should use the lowest dose for the shortest possible time.
Driving and using machines
Using this medication does not affect your ability to drive or use machines, as absorption is virtually zero or insignificant.
Rosalgin pronto contains benzalkonium chloride, ethanol, and fragrances that contain allergens
This medication contains 28 mg of benzalkonium chloride per vial (140 ml). Benzalkonium chloride may cause local irritation.
This medication contains 107.8 mg of ethanol per vial (140 ml). It may cause a burning sensation on damaged skin.
This medication contains fragrances with benzyl alcohol, benzyl benzoate, benzyl cinnamate, benzyl salicylate, citral, citronellol, eugenol, farnesol, geraniol, linalool, and d-limonene.
Benzyl alcohol, benzyl benzoate, benzyl cinnamate, benzyl salicylate, citral, citronellol, eugenol, farnesol, geraniol, linalool, and d-limonene may cause allergic reactions.
3. How to use Rosalgin pronto
Follow the administration instructions of the medication contained in this leaflet or as indicated by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist.
THIS MEDICATION IS USED ONLY VAGINALLY.
The recommended dose is 1 or 2 vaginal irrigations per day for 5 days.
Once the solution is applied, wash your hands with plenty of water.
If you are using other medications vaginally, Rosalgin pronto should be administered first to avoid eliminating the other medication.
It can be used at room temperature or by introducing the closed vial into warm water for a few minutes.
To use the single-dose applicator, follow these steps:
 1º To open the vial, keep the upper ring fixed, as shown in the figure, and turn the cap until the seal is broken.
1º To open the vial, keep the upper ring fixed, as shown in the figure, and turn the cap until the seal is broken.
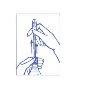 2º Extract the cannula until you feel the impact with the upper part. Only the complete extraction of the cannula will allow the liquid to come out.
2º Extract the cannula until you feel the impact with the upper part. Only the complete extraction of the cannula will allow the liquid to come out.
3º Gently insert the cannula into the vagina and compress the vial until it is empty.
The emptying can be gradual. A built-in valve prevents the solution from flowing back into the vial.
To achieve full therapeutic activity, the liquid should be kept in the vagina for a few minutes.
If you use more Rosalgin pronto than you should
No cases of overdose with Rosalgin pronto used vaginally have been observed.
In case of accidental oral ingestion of bencidamine hydrochloride, you may not have symptoms or may feel nausea, vomiting, abdominal pain, dizziness, visual hallucinations, or irritability, muscle weakness, tremors, drowsiness, headache, blurred vision, high blood pressure, among others. There is no specific antidote, but it usually resolves spontaneously once the drug is eliminated. Symptomatic treatment may be necessary.
If you accidentally ingest some of your medication, contact your doctor or pharmacist immediately for advice. You can also call the Toxicology Information Service, phone 91 562 04 20, indicating the name of the medication and the amount ingested.
If you forget to use Rosalgin pronto
Do not apply a double dose to make up for forgotten doses.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Rosalgin pronto can cause side effects, although not everyone will experience them.
Very rare side effects (less than 1 in 10,000 people):
- Sensitivity of the skin to sunlight (causing rash or sunburn), burning, itching in the application area.
Reporting side effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Rosalgin pronto
No special storage conditions are required.
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the packaging after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away through the sewers or in the trash. Deposit the packaging and medications you no longer need at the SIGRE point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Contents of the package and additional information
Composition of Rosalgin pronto
- The active ingredient is bencidamine (as bencidamine hydrochloride). Each vial contains 140 mg of bencidamine hydrochloride.
- The other components (excipients) are: benzalkonium chloride, disodium edetate, ethanol, polysorbate 20, rose oil (contains: benzyl alcohol, benzyl benzoate, benzyl cinnamate, benzyl salicylate, citral, citronellol, eugenol, farnesol, geraniol, linalool, and d-limonene) and purified water.
Appearance of the product and contents of the package
Rosalgin pronto is presented as a vaginal solution. The solution is clear and colorless and is packaged in polyethylene vials with 140 ml of solution.
Marketing authorization holder
ANGELINI PHARMA ESPAÑA, S.L.
c/ Antonio Machado, 78-80
3ª planta, módulo A-Edificio Australia
08840 Viladecans, Barcelona (España)
Manufacturer
A.C.R.A.F. SpA
Via Vecchia del Pinocchio, 22
60131 Ancona (Italia)
Date of the last revision of this leaflet:July 2025.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROSALGIN PRONTO 140 mg VAGINAL SOLUTIONDosage form: VAGINAL LIQUID, 500 mg benzydamine hydrochlorideActive substance: benzydamineManufacturer: Angelini Pharma Espana S.L.Prescription not requiredDosage form: TABLET, 20 mgActive substance: Agni casti fructusManufacturer: Bionorica SePrescription not requiredDosage form: INJECTABLE PERFUSION, 7.5 mg/mlActive substance: atosibanManufacturer: Altan Pharmaceuticals SaPrescription required
Online doctors for ROSALGIN PRONTO 140 mg VAGINAL SOLUTION
Discuss questions about ROSALGIN PRONTO 140 mg VAGINAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






