How to use Tantum Rosa
Leaflet attached to the packaging: patient information
TANTUM ROSA, 1mg/ml, vaginal solution
Benzydamine hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as advised by a doctor, pharmacist, or nurse.
- This leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If there is no improvement after 7 days or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Tantum Rosa and what is it used for
- 2. Important information before using Tantum Rosa
- 3. How to use Tantum Rosa
- 4. Possible side effects
- 5. How to store Tantum Rosa
- 6. Contents of the packaging and other information
1. What is Tantum Rosa and what is it used for
Tantum Rosa contains the active substance benzydamine, which belongs to non-steroidal anti-inflammatory drugs (NSAIDs).
Benzydamine has anti-inflammatory, analgesic, and local anesthetic and antiseptic properties. The medicine is well absorbed locally, achieving high concentrations in inflamed tissues. The medicine is generally well tolerated. It is used for the local treatment of inflammation symptoms.
Tantum Rosa is used vaginally to treat symptoms such as pain, burning, itching, redness, discharge, swelling in the external genital area and vagina.
These symptoms occur as a result of vulvitis and vaginitis, cervicitis of various origins (e.g., infections, mechanical irritation), also after chemotherapy and radiotherapy. The medicine is also used for pre- and post-operative prophylaxis in gynecology.
2. Important information before using Tantum Rosa
When not to use Tantum Rosa
If the patient is allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Tantum Rosa, the patient should discuss it with their doctor, pharmacist, or nurse.
Using, especially long-term, locally acting medicines may cause an allergic reaction. In such a case, Tantum Rosa should be discontinued and the patient should consult their doctor, who will recommend appropriate action.
Patients with genital bleeding or discharge should contact their doctor before using this medicine.
Tantum Rosa is intended for vaginal use. It should not be taken orally.
Tantum Rosa and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
No interactions between Tantum Rosa and other medicines used for the same indications are known.
Pregnancy and breastfeeding
In pregnancy and breastfeeding, or if pregnancy is suspected, or if the patient plans to become pregnant, they should consult their doctor or pharmacist before using this medicine.
Unless the doctor advises otherwise, the medicine should be used as recommended in the leaflet.
Driving and using machines
The medicine does not affect the ability to drive or use machines.
Tantum Rosa contains benzalkonium chloride and polysorbate 20 (E 432).
Benzalkonium chloride
The medicine contains 28 mg of benzalkonium chloride in each 140 ml solution, which corresponds to 0.2 mg/ml.
Benzalkonium chloride may cause local irritation.
Polysorbate 20 (E 432)
Polysorbates may cause allergic reactions.
The medicine contains a fragrant substance with: benzyl alcohol, benzyl benzoate, benzyl cinnamate, benzyl salicylate, citral, citronellol, eugenol, farnesol, geraniol, linalool, and limonene.
Benzyl alcohol, benzyl benzoate, benzyl cinnamate, benzyl salicylate, citral, citronellol, eugenol, farnesol, geraniol, linalool, and limonene may cause allergic reactions.
3. How to use Tantum Rosa
This medicine should always be used exactly as described in the patient leaflet or as advised by a doctor, pharmacist, or nurse.
In case of doubt, the patient should consult their doctor, pharmacist, or nurse.
The recommended dosage is 1 to 2 vaginal douches per day, depending on the severity of symptoms.
Treatment of symptoms and signs of inflammation of the external genital area (vulva and vagina) coexisting with vaginitis and/or cervicitis: douche the vagina with the solution 1 to 2 times a day, unless the doctor advises otherwise.
Pre- and post-operative prophylaxis in gynecology: the doctor will decide on the appropriate use of the medicine.
If the patient starts treatment on their own, they should not use the medicine continuously for more than 7 days without consulting their doctor.
The treatment should not last less than 3 days to achieve improvement.
The medicine is intended for vaginal douching. Each bottle is for single use. The vaginal solution, at room temperature, is ready for use. It is possible to warm it up by immersing the bottle in warm water for a few minutes.
Instructions for use:
- 1. Holding the bottle by the ring, twist the cap to break the seal, then remove the cap. (fig. 1)
Pull out the applicator tip until it stops or a characteristic click is heard.
Only full extension of the applicator allows the valve to be unlocked and the douching to be performed. (fig. 2)
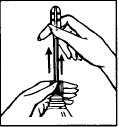

fig. 1
fig. 2
- 2. The patient should lie down or sit up, then insert the applicator tip into the vagina and slowly press the bottle walls until it is completely empty.
After the douching, the patient should remain lying down for a few minutes to retain the solution in the vagina.
Using more than the recommended dose of Tantum Rosa
Overdose of the medicine is unlikely due to the low concentration of the active substance and local route of administration. No cases of overdose with Tantum Rosa vaginal solution have been reported so far.
In case of accidental ingestion of the medicine, the patient should immediately contact their doctor or pharmacist.
If there are any further doubts about using the medicine, the patient should consult their doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Tantum Rosa can cause side effects, although not everybody gets them.
Side effects of unknown frequency (frequency cannot be estimated from the available data):
burning sensation at the site of application, photoallergic rash, urticaria.
Reporting side effects
If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will allow for more information to be collected on the safety of the medicine.
5. How to store Tantum Rosa
The medicine should be stored out of sight and reach of children.
Store in an upright position.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Tantum Rosa contains
The active substance of the medicine is benzydamine hydrochloride.
100 ml of the solution contains 100 mg of benzydamine hydrochloride.
The other excipients are: benzalkonium chloride solution, disodium edetate, ethanol 96%, polysorbate 20 (E 432), rose oil (benzyl alcohol, benzyl benzoate, benzyl cinnamate, benzyl salicylate, citral, citronellol, eugenol, farnesol, geraniol, linalool, and limonene), purified water.
What Tantum Rosa looks like and what the packaging contains
Tantum Rosa is a clear, colorless solution with a characteristic rose fragrance.
The immediate packaging of the medicine is a pink plastic bottle in the shape of a pear, equipped with a white applicator made of plastic with a valve and closure, containing 140 ml of the solution.
The applicator is protected with a pink plastic cap.
The outer packaging, a cardboard box, contains 1 or 5 bottles.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.p.A.
Viale Amelia 70, 00181 Rome, Italy
Manufacturer:
Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.p.A.
Via Vecchia del Pinocchio 22
60131 Ancona, Italy
Representative of the marketing authorization holder
Angelini Pharma Polska Sp. z o.o.
Aleje Jerozolimskie 181B
02-222 Warsaw
tel.: (22) 70 28 200
e-mail: [email protected]
The patient leaflet in a format suitable for the blind and visually impaired is available at the representative's office of the marketing authorization holder.
Date of the last update of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tantum RosaDosage form: Powder, 53.2 mg/gActive substance: benzydamineManufacturer: Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.p.A. Fine Food & Pharmaceuticals NTM S.P.A.Prescription not requiredDosage form: Concentrate, 37.5 mg/5 mlActive substance: atosibanPrescription not requiredDosage form: Solution, 6.75 mg/0.9 mlActive substance: atosibanPrescription not required
Alternatives to Tantum Rosa in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tantum Rosa in Ukraine
Alternative to Tantum Rosa in Spain
Online doctors for Tantum Rosa
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tantum Rosa – subject to medical assessment and local rules.